Munich, Germany (UroToday.com) Statins, also known as HMG-CoA reductase inhibitors, are a class of low molecular weight drugs which have been shown in preclinical models to demonstrate antitumor activity through apoptosis and cell growth arrest in three prostate cancer cell lines1. In a large prospective cohort study of 34,989 male health professionals in the United States, the age-standardized incidence rates of advanced prostate cancer were 38 per 100,000 person-years in current statin users and 89 per 100,000 person-years, with a relative risk of advanced disease of 0.51 (95% confidence interval [CI] = 0.30 to 0.86) for statin users2. Similar findings have been reported utilizing a database of patients from the Portland Oregon Veteran Affairs Medical Center where statin use was also associated with a reduction in prostate cancer risk (odds ratio = 0.38, 95% confidence interval: 0.21, 0.69)3. These studies suggest that long-term statin use may be associated with a reduced risk of prostate cancer.
This study utilized data from the phase III TROPIC trial and analyzed the impact of statin use in patients with metastatic castration-resistant prostate cancer (mCRPC). TROPIC studied the effect of cabazitaxel or mitoxantrone in men with mCRPC who had progressed on docetaxel4. 755 men were randomized to either cabazitaxel or mitoxantrone, and the hazard ratio for death of men treated with cabazitaxel compared with those who received mitoxantrone was 0.70 (95% CI 0·59–0·83, p<0·0001). Of the 755 patients in this study, 18.6% received some form of statin (138/755), approximately half in each arm.
Of note, patient characteristics were not balanced between patients receiving statins and those without. Patients on statins were older, had higher hemoglobin, lower alk phos, and lower rate of visceral metastases at the start of the trial.
In terms of response rates, PSA30 was higher with cabazitaxel and mitoxantrone for those patients on statins than those without statins. This was consistent for radiographic response rate for cabazitaxel but not for mitoxantrone.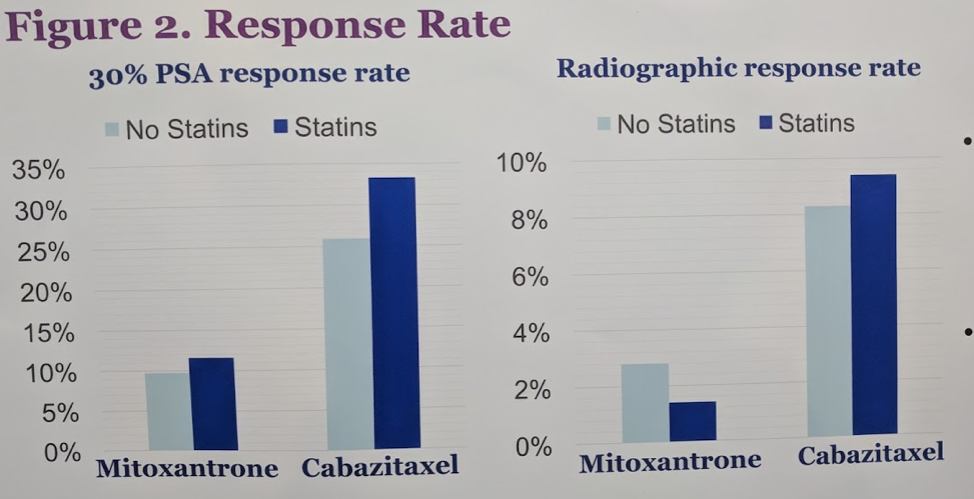
Patients who were on statins had a slightly longer median overall survival (15.8 vs 13.4m; HR: 0.74; p = 0.01) but no difference in PSA progression-free survival or radiographic progression-free survival.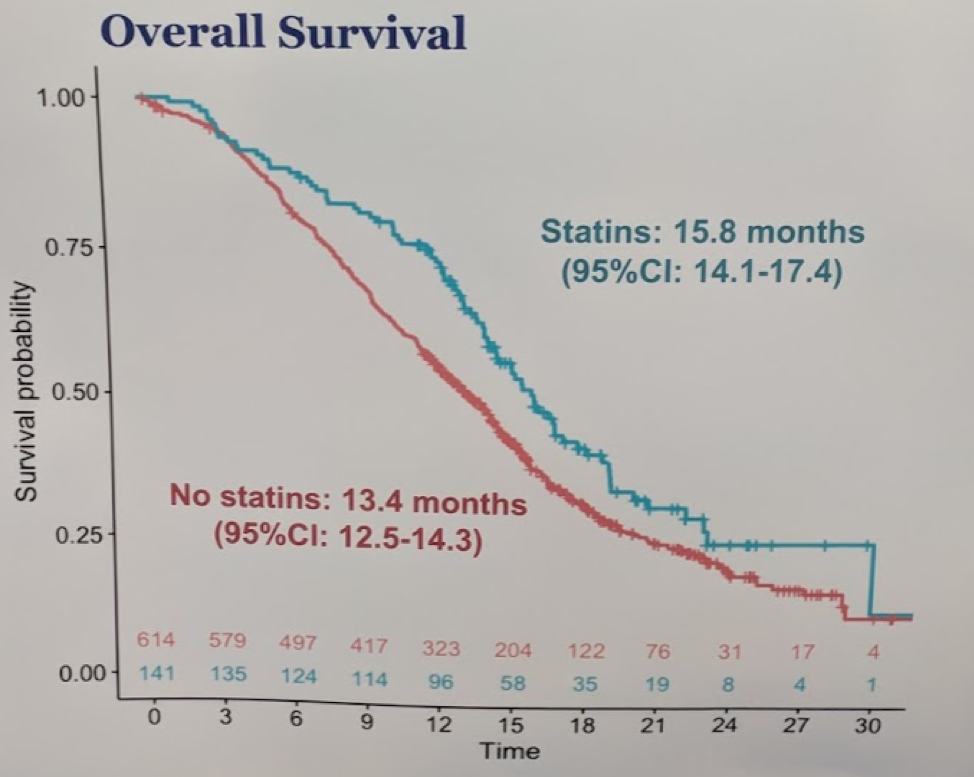
This study suggests that statin use in combination with cabazitaxel may improve outcomes compared with cabazitaxel alone. However, there are several limitations to this study. First, patient heterogeneity makes analysis of these results complicated as patients were on 5 types of statins (atorvastatin (53 pts, 38.4%), simvastatin (56 pts, 40.6%), rosuvastatin (14 pts, 10.1%), pravastatin (9 pts, 6.5%), lovastatin (4 pts, 2.9%) and fluvastatin (2 pts; 1.4%)) and it is unknown if anyone one statin in particular or the entire class of statins are associated with benefit. Second, it is unknown why patients were on the statins and perhaps the underlying reasons may be playing a role. Statins are used not only for the management of hyperlipidemia but also for coronary artery disease as well as cerebral vascular events. Analysis of these comorbid conditions may be important in future studies. Lastly, this is not a prospective, randomized trial. Future prospective randomized studies with statins may further shed light on their interesting results.
Presented by: David Lorente, MD, The Institute of Cancer Research and The Royal Marsden National Health Service Foundation Trust, Sutton, GB
References:
1. Hoque A, Chen H, Xu X-c. Statin Induces Apoptosis and Cell Growth Arrest in Prostate Cancer Cells. Cancer Epidemiology Biomarkers & Prevention 2008;17:88-94.
2. Platz EA, Leitzmann MF, Visvanathan K, et al. Statin Drugs and Risk of Advanced Prostate Cancer. JNCI: Journal of the National Cancer Institute 2006;98:1819-25.
3. Shannon J, Tewoderos S, Garzotto M, et al. Statins and Prostate Cancer Risk: A Case-Control Study. American Journal of Epidemiology 2005;162:318-25.
4. De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. The Lancet 2010;376:1147-54.
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


