In the TAX 327 study, docetaxel chemotherapy was shown to improve median overall survival in mCRPC patients by 2.9 months.1 This significant result was demonstrated despite the several limitations, including the fact that docetaxel was compared to alternative effective treatment (mitoxantrone) in this study. Additionally, there was a 30% crossover in this study, and the design was not ideal, as the first PSA was tested at six weeks following treatment. In a similar Chinese study, designed almost identically to TAX 427, docetaxel conferred an overall survival benefit of 8 months (21.9 vs. 13.7 months, the hazard ratio of 0.63).2
During the years 2004-2016, there have been several phase-3 trials conducted in the setting of mCRPC patients, demonstrating significant survival advantages for the novel treatments analyzed (Figure 1).

Figure 1 – Phase 3 trials during 2004-2016 in mCRPC patients:
During the last seven years, three significant paradigm changes in the management of prostate cancer have occurred:
- In 2011 – Novel androgen receptor agents were first given before chemotherapy in mCRPC patients
- In 2014 docetaxel was given in addition to ADT in men presenting with M1 hormone-sensitive disease (mHSPC)
- In 2017 abiraterone had been added to ADT in men with mHSPC
FIRSTANA was a first line phase 3 study randomizing mCRPC patients with no prior chemotherapy to either docetaxel, cabazitaxel 20 mg, or cabazitaxel 25 mg.6 The overall survival was similar among three arms with no significant difference. In the FIRSTANA trial, very few patients were previously treated with abiraterone or enzalutamide (<2%). Therefore, this trial did not inform us of the efficacy of Cabazitaxel compared to docetaxel in patients progressing after treatment with abiraterone or enzalutamide. Dr. De Wit’s strategy is to give cabazitaxel if there is no benefit during the first three months of being treated with docetaxel, in patients who had progressed on abiraterone or enzalutamide.
In the TROPIC study, the efficacy and safety of cabazitaxel plus prednisone was compared to that of mitoxantrone plus prednisone in men with mCRPC with progressive disease after docetaxel-based treatment. 7 74% of the men in this study had disease progression during or within three months after docetaxel. The median overall survival in these patients was 13.8 vs. 10.9 months, with a hazards ratio of 0.7, favoring cabazitaxel.7
For patients with a response duration of more than 12 months while on ADT and docetaxel, a reasonable option would be androgen receptor targeted therapy. In contrast, if the response duration was less than 12 months, the next choice should be cabazitaxel, according to Dr. De Wit. Data on Cabazitaxel gave as a 2nd line and 3rd line therapy has shown that as the 2nd line it entails a median overall survival of 20 months, while for the 3rd line, it entails an overall survival of 12.5 months.
Summarizing these data, to date, there are multiple options and sequences. In mHSPC early taxane/abiraterone or late taxane or abiraterone, are all viable options. In mCRPC abiraterone can be given before or after docetaxel and before or after cabazitaxel. Lastly, Radium 223 can be given following abiraterone/enzalutamide and before or after chemotherapy. New avenues that should be researched further include PSMA guided therapy, molecularly targeted therapies, and immunotherapy.
177-Lutetium -PSMA targeted therapy was the next topic discussed. PSMA is a transmembrane protein expressed in aggressive variants of prostate cancer. ADT upregulates it and second-generation androgen receptor targeted therapy. Radio-conjugated PSMA antagonists, such as 68 Ga and 177 Lutetium-PSMA bind to the cell membrane PSMA receptors and undergo internalization. Following internalization in the cell, the beta or alpha units exert a toxic effect on the tumor DNA. There are currently no randomized data regarding the efficacy of Lutetium. In the ASCO 2018 meeting, an abstract was presented, which was a phase 2 trial, including 50 patients with PSMA avid mCRPC patients, who progressed after conventional therapies. These patients received up to 4 cycles of Lutetium-PSMA every six weeks with the primary endpoint of PSA reduction by more than 50%. In 62% of these patients, the primary endpoint was reached with median overall survival of 12 months.8 90% of these patients were previously treated with abiraterone or enzalutamide, 84% were previously treated with docetaxel, and 48% were previously treated with docetaxel and cabazitaxel.
Despite the great improvement in PSMA scans following Lutetium treatment, the overall median survival was only 12 months. To explain this discrepancy, Dr. De Wit suggests that prostate cancer contains both PSMA positive and PSMA negative clones. Therefore, Lutetium may only be targeting part of the disease (the PSMA negative clones manage to escape).
The next topic discussed was the role of liquid biopsy in mCRPC patients. For liquid biopsy, either circulating tumor cells (CTC) or cell-free DNA (cfDNA) can be used. A concise table comparing these two different tests was shown (Table 1). Dr. De Wit believes that it is not prime time yet to use liquid biopsy tests in a real-world clinical application.
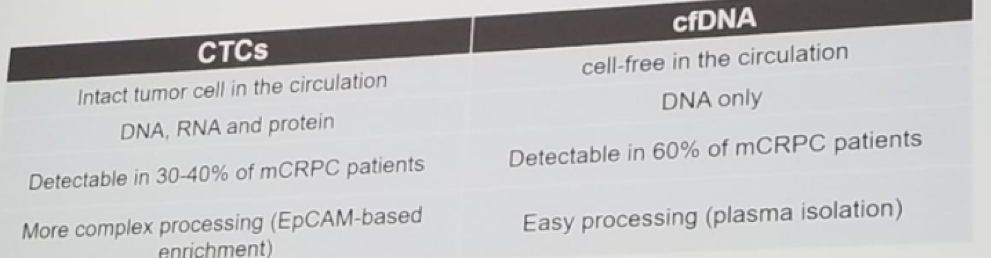
Table 1 – Comparison between circulating tumor cells and cell-free DNA:
The last topic discussed was the novel role of immunotherapy in the mCRPC setting. The Keynote-199 study analyzed the role of Pembrolizumab for post-docetaxel mCRPC patients.10 The study design is shown in figure 2, demonstrating that there were three planned cohorts, one with PD-L1 positive tumors only, one with PD-L1 negative tumors only, and the third cohort with bone metastases and any PD-L1 status. Both PD-L1 positive and PD-L2 negative patients had PSA responses. 10% of patients had a PSA decline between 30% to 100% from baseline. When combining all three cohorts, 11% of patients had a PSA decline greater than 50%. When analyzing the radiographic responses, in the PD-L1 positive population, 2% of the patients had a complete response, while 4% of the patients had only a partial response. In the PD-L1 negative, population, similar results were shown, with a 0% complete response. Summarizing the results, pembrolizumab demonstrated anti-tumor activity in a very small proportion of patients, and PD-L1 status alone, did not appear to differentiate responders from non-responders.

Figure 2 – Keynote 199 trial design:
Concluding his detailed talk, Dr. De Wit, recommended the combination of taxanes with androgen receptor targeted therapies for mCRPC patients. To date, there is no accepted standard regarding what kind of combination to use, and it only occurs as part of a clinical trial. There have been several trails that have already published their results, and there are currently several ongoing phase 1-2 trials assessing some combinations, as can be seen in figure 3.

Figure 3 – Several concluded and ongoing phase 1-2 trials assessing the combination of taxanes and androgen receptor targeted therapy:
In summary, the management of mCRPC has greatly evolved in the last 14 years. Many new agents have been incorporated as part of the treatment strategy, and some questions remain about the appropriate sequencing and combination of these agents.
Presented by: Ronald De Wit, MD, Ph.D., Professor, Rotterdam, the Netherlands
References:
1. Tannock et al. NEJM 2004
2. Ti Zou et al. Plos one 2015
3. PLATO Attard et al. JCO 2018
4. Van Soest et al. Eur Urol 2014
5. De Bono et al. Eur Urol 2017
6. Sartor O et al. ASCO 2016
7. Loriot Y et al. Eur J Cancer 2015
8. De Bono JS et al. LANCET 2010
9. Sandhu et al. ASCO 2018
10. De Bone JS ASCO 2018
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany
Further Related Content:
Oligometastatic Disease - The Critical Need of a Multidisciplinary Approach for the Treatment of Prostate Cancer


