This abstract examines data from PROSPER by race and region. The trial design of PROSPER is shown below:
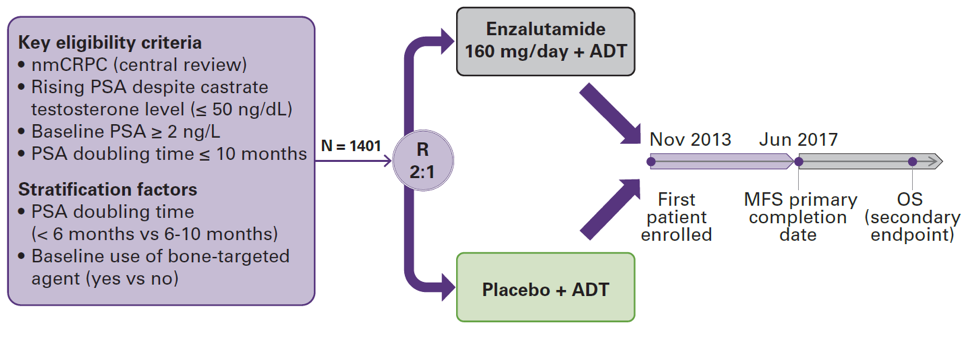
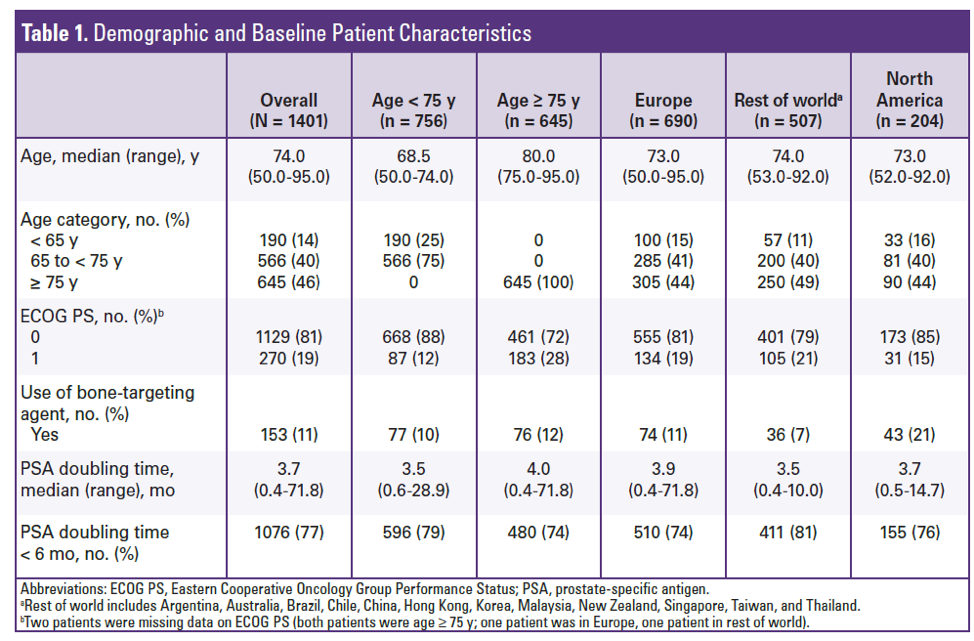
They randomized 1401 patients to receive either enzalutamide or placebo. The trial was initiated in 2013 and met its MFS primary completion day in June of 2017. In terms of baseline characteristics, the median age of those in the <75 cohort was 68, and the median age of patients >75 was 80. Most of the patients <75 were between 65-75, with only 25% of patients less than 65. There were more patients with ECOG of 0 in the <75 cohort (88% vs 72%). The use of bone targeting agents was similar in both arms. PSA doubling time was not significantly different between any cohort.
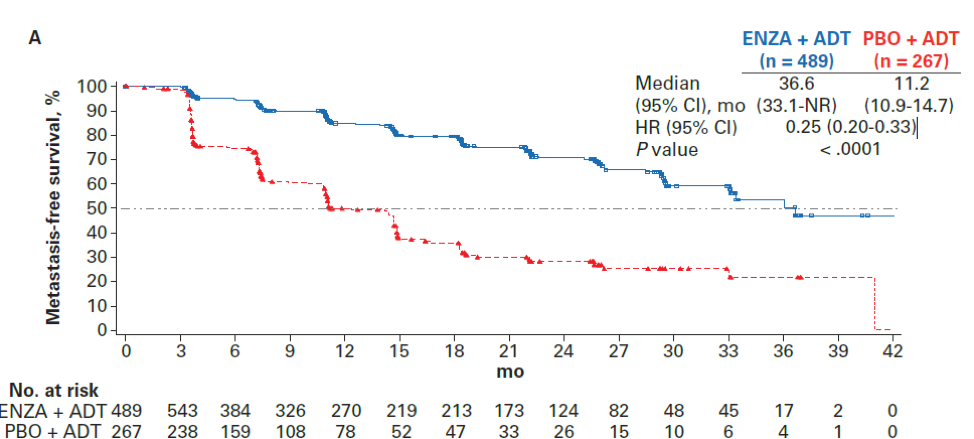
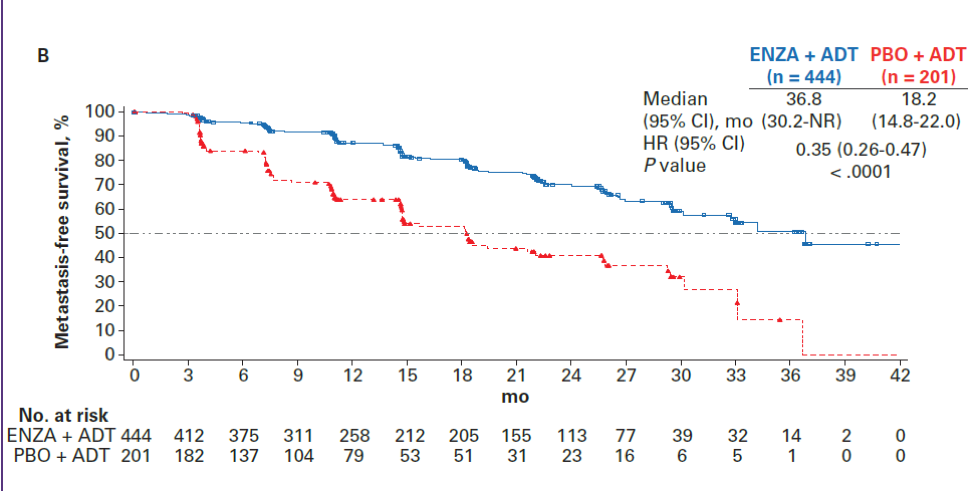
In terms of efficacy, enzalutamide + ADT demonstrated a benefit over placebo + ADT in both the age<75 and age≥75 cohort. For patients younger than 75, MFS was 36.6 months compared with 11.2 months (HR 0.25, 95%CI 0.20-0.33, p<0.0001). For patients older than 75, MFS was 36.8 months with enzalutamide compared with 18.2 months with placebo (HR 0.35, 95%CI 0.26 – 0.47, p<0.0001). Panel A below shows MFS for patients <75 and panel B shows MFS for patients >75.
Metastasis-free survival benefit was seen regardless of where the therapy was delivered as well, with respect to North America, Europe, and “Rest of World” which included Argentina, Australia, Brazil, Chile, China, Hong Kong, Korea, Malaysia, New Zealand, Singapore, Taiwan, and Thailand.
In terms of tolerability, patients 75 or older had a higher rate of discontinuation, which is not surprising given the toxicity profile of enzalutamide (falls, fatigue).
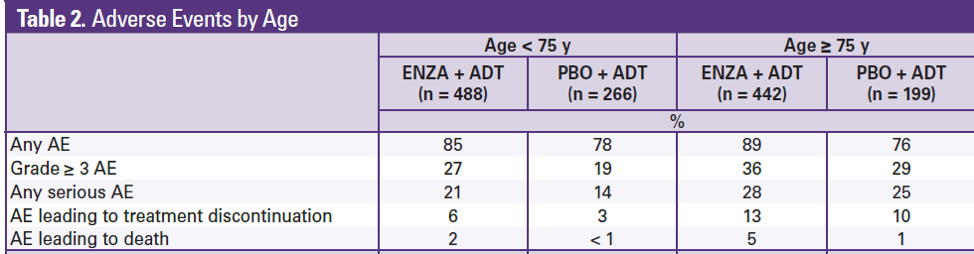
This abstract shows that in patients with M0 CRPC with a PSA doubling time of less than 10 months, enzalutamide is reasonable to use in older patients (≥75 years old) and well tolerated. The original registration trial led to the FDA approval of enzalutamide in for M0CRPC in the United States. For patients ≥75 years old, fatigue (33%) and falls (16%) are common and assessment of patient frailty will be important before prescribing enzalutamide, especially in those older than 75.
Presented by: Karim Fizazi, MD, Ph.D., Medical Oncologist, Head of the Department of Cancer Medicine at the Institut Gustave Roussy, Villejuif, France and Professor in Oncology at the University of Paris
References:
1.Hussain M, Fizazi K, Saad F, et al. PROSPER: A phase 3, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) in men with nonmetastatic castration-resistant prostate cancer (M0 CRPC). American Society of Clinical Oncology; 2018.
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


