Barcelona, Spain (UroToday.com) Given that targeted therapies and immunotherapy have different mechanisms of action, it is reasonable to consider that the combination of targeted therapy with immunotherapy would achieve superior clinical outcomes relative to either modality alone. At a special symposium of the immune-oncology landscape for first-line treatment of metastatic urothelial cancer, Dr. Yohann Loriot summarized the available data on combinations of immunotherapy with molecular targeted therapy.
There are data to suggest that targeted therapy may induce immunogenic cell death, leading to upregulation of MHC class I, increased antigen presentation and T lymphocyte proliferation, as well as a compensatory increase in PD-L1 expression. These changes within the tumor microenvironment may suggest an induced susceptibility to checkpoint blockade immunotherapy.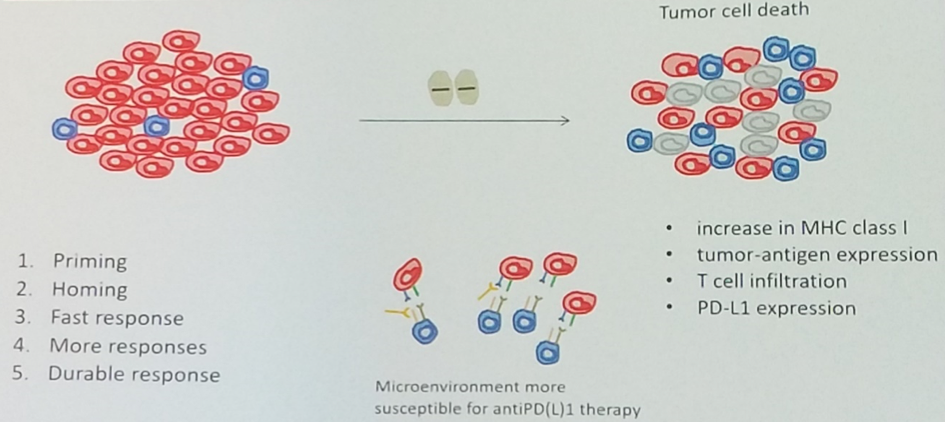
Dr. Loriot moved on to highlight ways in which targeted therapies are known to leverage the native antineoplastic immune response. Just as cytotoxic chemotherapy and radiation are able to induce immunogenic cell death, so too are molecularly targeted therapies. Specifically, targeted therapy has been shown to alter the immune landscape of the microenvironment so as to increase the frequency of CD8+ T lymphocytes and decrease the frequency of myeloid-derived suppressor cells and regulatory T cells.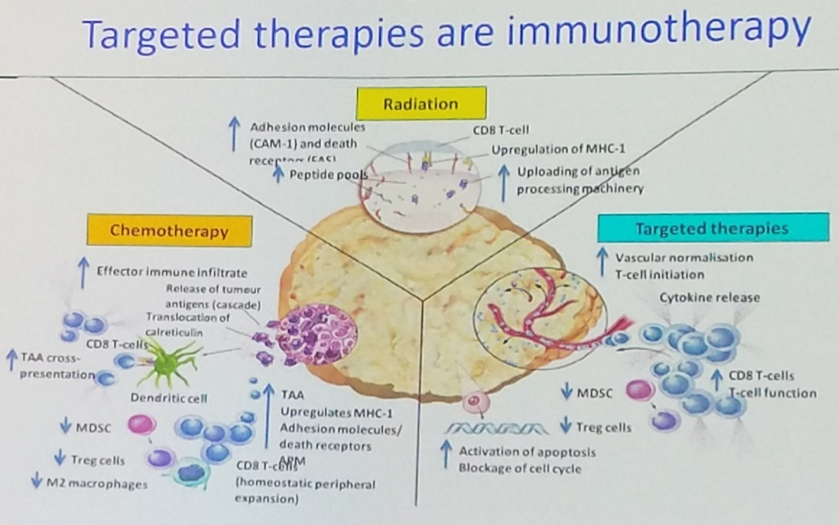
Dr. Loriot used available data from BRAF and MEK inhibition in melanoma to identify mechanisms by which targeted therapy alters the composition of the tumor microenvironment in such a way as to prime the tumor for an immune response. Data suggest that BRAF monotherapy is associated with an increase in antigen expression and CD8+ T cell infiltration. However, MEK inhibition has been associated with impairment of the antineoplastic T cell response, and triplet combinations with BRAF and MEK inhibition plus an immune checkpoint inhibitor have been plagued by high rates of toxicity. Fortunately, more recent data on the combination of vemurafenib plus cobimetinib with the PD-L1 antibody atezolizumab was able to mitigate much of this toxicity while preserving efficacy primarily by administering these agents in sequence rather than concomitantly.
Dr. Loriot then moved on to discuss immune-oncology combinations specific to metastatic urothelial cancer. Given the luminal-papillary molecular subtype which is enriched for FGFR3 alterations and exhibits an immunologically “cold” phenotype, there is much interest in combining FGFR blocking agents with immune checkpoint inhibition. FIERCE-22 is a phase I/II study of the FGFR3 antibody vofatamab in combination with the PD-1 antibody pembrolizumab in unselected patients with platinum-refractory metastatic urothelial cancer. The combination achieved a 36% objective response rate, and interestingly, among 7 patients harboring FGFR3 mutations or fusions, 3 (43%) had an objective response.
Furthermore, on-treatment biopsies from FIERCE-22 demonstrated that vofatamab was capable of inducing an immune gene expression signature, lending support to the underlying biologic rationale.
Given the recent accelerated FDA approval of the pan-FGFR inhibitor erdafitinib, a number of trials are underway to investigate the safety and efficacy of combining erdafitinib and other FGFR antibodies with immunotherapy. Specifically, NORSE is a phase Ib/II study of FGFR2/3 altered cisplatin-ineligible metastatic urothelial cancer investigating erdafitinib with or without the PD-1 antibody cetrelimab.
Still, other trials are exploring the combination of antiangiogenic agents, PARP inhibitors, and HER2 directed therapy with checkpoint blockade.

In conclusion, a number of novel immune-oncology combinations are exploring the efficacy of various targeted therapies and checkpoint blockade immunotherapy in metastatic urothelial carcinoma. Future work will focus on identifying the most effective targeted agents to combine with immunotherapy and defining the optimal sequencing of therapies to augment the immune response and mitigate toxicity associated with combination regimens.
Presented by: Yohann Loriot, MD, PhD, Medical Oncologist, Gustave Roussy Institute, Villejuif, France
Written by: Michael Lattanzi, MD, Medical Oncology Fellow, Memorial Sloan Kettering Cancer Center, Twitter: @MikeLattanzi at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain


