Barcelona, Spain (UroToday.com) The STAMPEDE trial consortium previously reported that upfront docetaxel improved overall survival (OS) for patients starting long-term androgen deprivation therapy (ADT).1 Over a median follow-up of 43 months (IQR 30–60), there were 415 deaths in the control group, with a median OS of 71 months (IQR 32-not reached (NR)) for standard of care, NR (IQR 32-NR) for standard of care + zoledronic acid (HR 0.94, 95%CI 0.79–1.11), 81 months (41-NR) for ADT + docetaxel (HR 0.78, 95%CI 0.66–0.93), and 76 months (IQR 39-NR) for standard of care + zoledronic acid + docetaxel (HR 0.82, 95%CI 0.69–0.97). At the ESMO 2019 prostate cancer session, Professor Nick James presented the long-term outcomes for M1 patients using OS as the primary outcome measure. The second objective was to assess if the benefit of docetaxel depended on metastatic burden, as suggested by previous trials,2 using the CHAARTED definition of high burden and low burden baseline disease.
For this study, there were 724 patients receiving standard of care and 362 patients receiving standard of care + docetaxel that were recruited with a 2:1 randomized stratified allocation.
The full study design, with excluded patients, is as follows: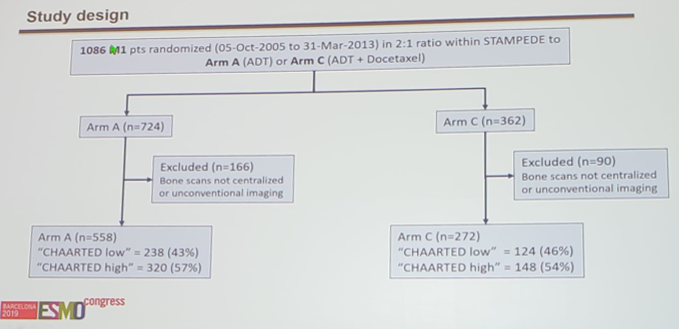
The analysis used Cox regression models, adjusted for all stratification factors, with emphasis on restricted mean survival time if hazards were non-proportional. Retrospectively-collected imaging data, blinded to trial arm, was used to categorize patients as having a low- or high-burden disease.
Median follow-up was ∼6.5yr, compared to ∼3.5yr when last reported. There were 494 deaths on standard of care (41% increase in deaths compared to the previous report), with median OS = 43.1 months. There was evidence of the benefit of standard of care + docetaxel on OS (median = 59.1 months, HR 0.81, 95% CI 0.69-0.95, p = 0.009).
Metastatic burden was assessable for 830/1086 (76%) patients - subgroups were representative of the full M1 cohort in terms of stratification factors. There was no evidence of heterogeneity of docetaxel effect between the low-burden and high-burden subgroups (interaction p = 0.827; low-burden HR 0.76, 95% CI 0.54-1.07, p = 0.107; high-burden HR 0.81, 95% CI 0.64-1.02, p = 0.064).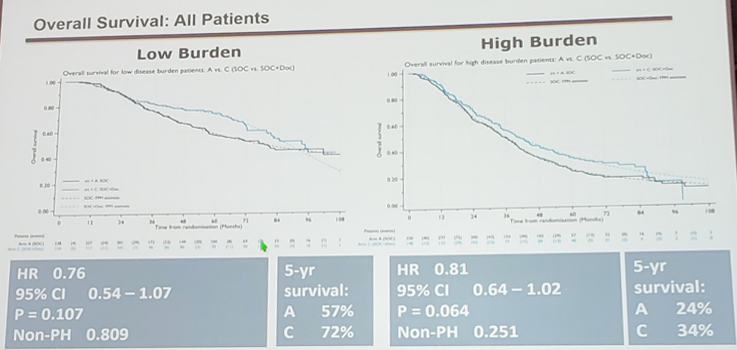
Analysis of other outcomes also found evidence of benefit of standard of care + docetaxel over standard of care in failure-free survival (FFS HR 0.66, 95% CI 0.57-0.76) and progression-free survival (PFS HR 0.69, 95% CI 0.59-0.81), and no evidence of heterogeneity of docetaxel effect between metastatic burden subgroups for any outcome (FFS: p = 0.792; PFS: p = 0.855; PCSM: p=0.413).
There was no evidence that standard of care + docetaxel resulted in late (after 1yr) grade 3-5 toxicity compared to standard of care (27% vs 28% respectively).
Dr. James suggests that this analysis does not support the presence of a volume effect on docetaxel effect in men with newly diagnosed metastatic prostate cancer, although metastatic burden is prognostic. He notes that STAMPEDE has almost exclusively newly diagnosed patients, which allows for a better power to assess effects as a homogenous group, and suggests that differences with GETUG-15 and CHAARTED may be related to high proportions of relapsed patients in low metastatic burden groups in both trials. The absolute gain in 5-year survival for low metastatic burden patients is 57% à 72% and for high metastatic burden is 24% à 34%.
Dr. James concluded with several points:
- Docetaxel + ADT improves OS and FFS in newly diagnosed mHNPC patients regardless of disease burden.
- He suggests that this data is consistent with data for abiraterone, enzalutamide, and apalutamide.
- Docetaxel should now be considered as a first-line option alongside AR targeting agents for all de novo mHNPC patients regardless of stratification for “disease burden.”
Presented by: Professor Nicholas James BSc, MB, BS, FRCP, FRCR, Ph.D., Consultant in Clinical Oncology at the Queen Elizabeth Hospital Birmingham and Professor of Clinical Oncology at the University of Birmingham, NHS Foundation Trust, Birmingham, United Kingdom. Professor Nicholas James, presenting on behalf of Noel Clarke, MBBS, FRCS, ChM, FRCS, Professor of Urological Oncology, The Christie and Salford Royal Hospitals, Manchester, United Kingdom
Co-authors: A. Ali,1 F. Ingleby,2 A. Hoyle,1 J. Calvert,2 G. Attard,3 S. Chowdhury,4 D. Dearnaley,5 H. Douis,6 S. Gillessen,7 R. Jones,8 Z. Malik,9 M. Mason,10 R. Millman,11 C. Parker,12 H. Rush,2 A. Omlin,13 M. Sydes,2 M. Parmar,2 N. James14
1. The Christie and Salford Royal Hospitals, Manchester, UK
2. MRC Clinical Trials Unit at UCL, London, UK
3. The Institute of Cancer Research (Sutton Site), Sutton, UK
4. Guy's and St. Thomas' Hospital NHS Trust, London, UK
5. The Institute of Cancer Research (ICR), London, UK
6. University Hospital Birmingham, Birmingham, UK
7. The Christie NHS Foundation Trust, Manchester, UK
8. University of Glasgow, Glasgow, UK
9. Royal Liverpool University Hospital, Liverpool, UK
10. Velindre Cancer Centre Velindre Hospital, Cardiff, UK
11. Medical Research Council - MRC Clinical Trials Unit, London, UK
12. The Institute of Cancer Research/Royal Marsden NHS Foundation Trust, Sutton, UK
13. Kantonsspital St. Gallen, St. Gallen, CH
14. Queen Elizabeth-University Hospital Birmingham NHS Foundation Trust, Birmingham, UK
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain
References:
1. James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163-77.
2. Gravis G, Boher JM, Chen YH, et al. Burden of Metastatic Castrate Naïve Protate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur Urol 2018 Jun;73(6):847-855.
James, N., Ingleby, F., Clarke, N., Amos, C., Attard, G., & Cross, W. et al. (2019). Abstract 855PD Docetaxel for hormone-naïve prostate cancer (PCa): Results from long-term follow-up of non-metastatic (M0) patients in the STAMPEDE randomised trial. Annals Of Oncology, 30 (Supplement_5). doi: 10.1093/annonc/mdz248.008a,


