Barcelona, Spain (UroToday.com) PD-L1 is overexpressed by dendritic cells of mCRPC patients progressing on androgen receptor antagonist therapy. Interestingly, anti-PD-1 agents lead to infrequent but durable responses. Combination of anti-PD-L1 and anti-CTLA-4 is a promising approach because of the non-redundant pathway blockade and synergy based on preclinical data, as well as emerging clinical data. In the phase II CCTG IND 232 trial, the investigators, led by Dr. Sebastien Hotte, tested the hypothesis that dual checkpoint blockade of CTLA-4 with tremelimumab and PD-L1 with durvalumab enhances immune mediated activity in mCRPC.
CCTG IND 232 was a multicenter randomized phase II study of mCRPC patients with measurable disease, having received prior abiraterone and/or enzalutamide, and no more than one taxane for mCRPC. Patients were then randomized to durvalumab 1500mg IV Q4 weeks +/- 4 doses of tremelimumab 75mg IV. The primary endpoint was ORR (RECIST1.1 and iRECIST) using a Simon 2-stage design. The key secondary endpoints were PSA response rate and time to progression. A treatment arm would be considered of interest if there were ≥ 4 objective responses. Correlative testing is ongoing for PD-L1/CD8 IHC on mandatory tumour biopsies and a 74-gene panel sequencing of plasma cell-free DNA, both collected at baseline.
There were 52 patients enrolled in this trial, with a median age 70 years (range 50-83 years), 33% of patients were ECOG 0 or 1, and 48% of patients receiving prior taxane chemotherapy for CRPC. Among 13 patients randomized to durvalumab, none had an ORR; median time to progression was 2.1 months (95% CI 1.4-2.6). There were 37 patients that received durvalumab + tremelimumab who received a median of 3 cycles (range 1-27). Among these patients, there were six objective responses (16%), with an objective response median duration not reached, and the longest ongoing for more than 25 months (PSA < 0.2 ng/ml). All six patients remain on durvalumab and pain improved in 75% of patients. PSA response was also noted in 16% of patients, with a median time to progression of 2.6 months (95% CI 1.8-2.8 months).
Tumor response by treatment arm (arm 1 – durvalumab + tremelimumab; arm 2 – durvalumab)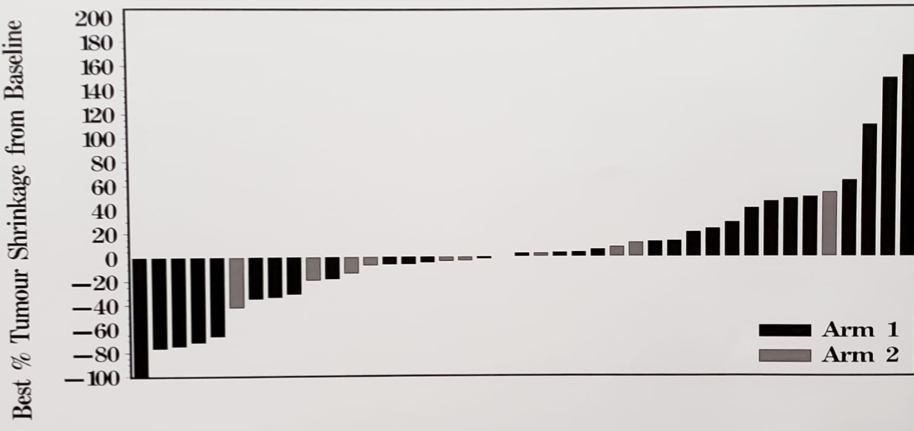
Durvalumab + tremelimumab related adverse events were mainly grade 2 or less: fatigue (46%), anorexia (28%), rash (24%), diarrhea (23%), nausea/vomiting (18%) and thyroid dysfunction (15%). The most common grade 3/4 adverse events were: increased LFTs (8%) and diarrhea (8%). Six pts discontinued treatment due to adverse events and there were no grade 5 adverse events.
The conclusions of CCTG IND 232 are as follows:
- Based on prespecified criteria, durvalumab did not show sufficient clinical activity
- Further studies incorporating patient selection by biomarkers are warranted for durvalumab + tremelimumab
- Future analysis will incorporate correlative testing for PD-L1/CD8 IHC and sequencing of plasma cell-free DNA
NCT02788773.
Presented by: Sebastien J. Hotte, MD, MSc (HRM), FRCPC, Medical Oncologist, Associate Professor, Department of Oncology, Juravinski Cancer Centre, Hamilton, Ontario, Canada
Co-authors: E. Winquist,2 K. Chi,3 S. Ellard,4 S. Sridhar,5 U. Emmenegger,6 M. Salim,7 N. Iqbal,8 C. Canil,9 C. Kollmannsberger,3 A. Hansen,10 A.-K. Lalani,11 J. Gingerich,12 D. Finch,13 M. Cabanero,14 A. Wyatt,15 D. Tu,16 F. Vera-Badillo,16 L. Seymour,17 M. Smoragiewicz16
2. London Regional Cancer Program (LRCP) - London Health Science Center (LHSC), London, CA
3. BC Cancer Agency - Vancouver, Vancouver, CA
4. BC Cancer Agency, Kelowna, CA
5. Princess Margaret Hospital, Toronto, CA
6. Sunnybrook Odette Cancer Center, Sunnybrook HSC, Toronto, CA
7. Saskatchewan Cancer Agency-Allan Blair Cancer Centre at Pasqua Hosp, Regina, CA
8. Saskatoon Cancer Centre University of Saskatchewan, Saskatoon, CA
9. The Ottawa Hospital Regional Cancer Centre, Ottawa, CA
10. Princess Margaret Cancer Center, Toronto, CA
11. Dana-Farber Cancer Institute, Boston, US
12. CancerCare Manitoba, Winnipeg, CA
13. BC Cancer Kelowna, Kelowna, CA
14. University Health Network - University of Toronto, Toronto, CA
15. University of British Columbia, Vancouver, CA
16. Queen's University, Kingston, CA
17. Canadian Cancer Trials Group, Kingston, CA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain


