In this poster, Dr. Graff and colleagues present data from a 30-patient expansion cohort (NCT02312557) of patients progressing on enzalutamide who then received pembrolizumab 200 mg IV every three weeks while continuing on enzalutamide. The initial study leading to this cohort was presented at ASCO 2019 and reported 18% durable responders from 28 patients studied with the same regimen. The primary endpoint of this study was a PSA decline of greater than 50%, and secondary endpoints were objective response, PSA PFS, radiologic PFS, overall survival, and single-cell transcriptomic as well as microbiota studies.
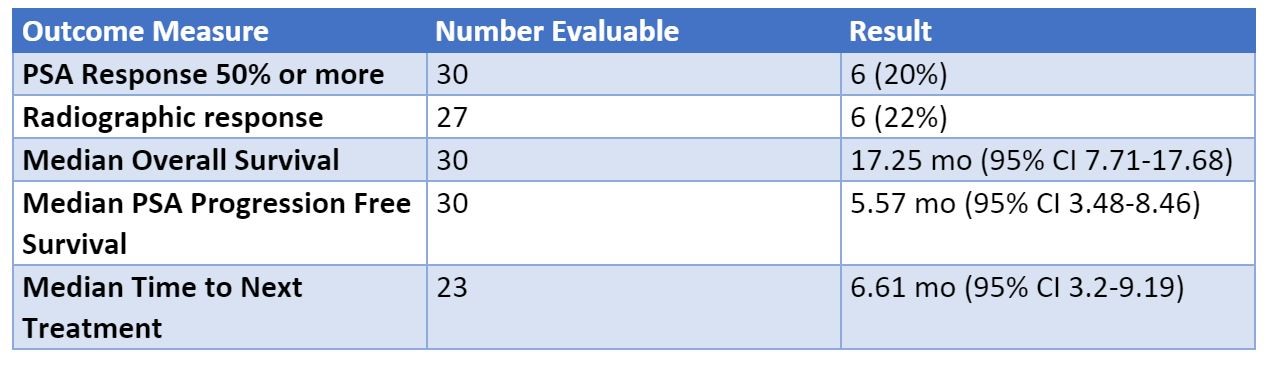
Overall there were six patients that met primary endpoint criteria of PSA response, and 22% of patients with evaluable disease showed an objective radiographic response. Eight patients had immune-related adverse events. Using single-cell transcriptomic profiling of patient samples obtained by rectal swab prior to pembrolizumab therapy, the authors identified a transcriptomic profile of “exhausted CD8 T cells” that correlated with response. Using bacterial profiling, the authors found lower levels of Akkermansia muciniphila organisms were also associated with treatment response.

These results suggest that pembrolizumab can rescue enzalutamide progression in a subset of patients. Potential biomarkers of this response include a “CD8 exhaustion” transcriptional signature in T-cells, and lower baseline abundance of Akkermansia muciniphila in patient fecal samples. Further prospective validation will be needed to ascertain the relevance of these biomarkers in predicting response to immunotherapy in mCRPC.
Presented by: Julie Graff, MD, Medical Oncologist, Knight Cancer Institute at Oregon Health & Science University and Chief of Hematology/Oncology, VA Portland Health care System, Portland, Oregon
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain


