Barcelona, Spain (UroToday.com) Prostate radiotherapy with androgen deprivation therapy (ADT) is now recommended as a first line option for de-novo low burden metastatic prostate cancer. In the STAMPEDE “M1|RT comparison” metastatic burden was a determinant of benefit, based on pre-specified prognostic criteria.1 However, the pre-specified metastatic burden criteria used to dichotomize was based on prognostic factors from the systemic therapy era. At the prostate cancer ESMO 2019 session, Dr. Ali and colleagues from the STAMPEDE group reported results of their exploratory analyses of metastases as defined by site and number to improve prediction of treatment benefit from prostate radiotherapy.
Patients randomized to the ADT (± docetaxel) vs prostate radiotherapy + ADT (± docetaxel) were studied in this analysis. 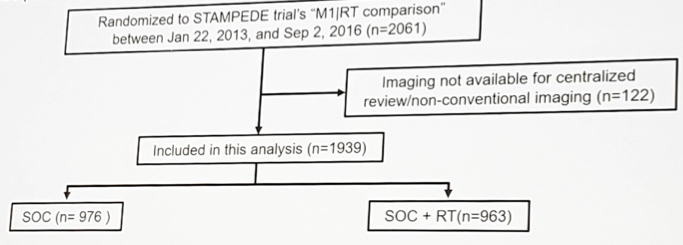
Metastatic site, distribution and number were evaluated based on conventional imaging and used to explore treatment effects to refine the metastatic burden definition. Results focused on the trial’s key outcome measures: overall (OS) and failure-free survival (FFS), analyzed using standard survival analysis methods. A hazard ratio < 1 indicates benefit associated with prostate radiotherapy + ADT (±docetaxel) over ADT (±docetaxel).
Among the 1,939 patients included, 181 patients had only lymph node mets, 1,587 had bone (± lymph node) mets, and 171 had other visceral mets (± bone/lymph node). Baseline characteristics such as age (median 68 years), PSA (median 98 ng/ml) were balanced between the arms. In lymph node only patients, prostate radiotherapy improved OS (HR 0.62, 95% CI 0.35-1.09) and FFS (HR 0.64, 95% CI 0.43-0.96). 
In bone (± lymph node) patients with <4 bone mets regardless of bone metastatic location, prostate radiotherapy improved OS (HR 0.65, 95% CI 0.47 – 0.92) and FFS (HR 0.58, 95% CI 0.46 – 0.73). However, there was no such evidence of benefit found in patients with visceral metastases (OS: HR 0.92, 95% CI 0.58 – 1.45) or bone (± lymph nodes) patients with ≥4 bone metastases (OS: HR 1.11, 95% CI 0.92 – 1.33). In the refined low met burden subgroup of patients with only lymph node or < 4 bone metastases (± lymph nodes), prostate radiotherapy improved OS (HR 0.62, 95% CI 0.46 – 0.83) and FFS (HR 0.57, 95% CI 0.47 – 0.70). Within the low met burden subgroup there was no evidence of heterogeneity in OS and FFS (all interaction p-values >0.1) for baseline factors such as age, N stage, Gleason score, radiotherapy schedule or docetaxel use.
The conclusions from this study were as follows:
- The data suggests that prostate radiotherapy + standard of care benefits patients with only non-regional lymph node metastasis but not patients with visceral metastasis over standard of care alone
- Bone metastasis counts based on conventional bone scan are predictive of OS and FFS benefit associated with prostate radiotherapy + standard of care regardless of anatomic location – improves FFS and OS in patients with <= 3 bone metastasis, there is a watershed at 4 bone metastasis, and beneficial effect at >=5 bone metastasis is less certain
- In an exploratory subgroup analysis, prostate radiotherapy + standard of care improved OS and FFS in men with only non-regional lymph nodes or <4 bone metastasis ((± lymph node) regardless of location and no visceral metastasis over standard of care alone
Clinical trial identification NCT00268476
Presented by: Syed Adnan Ali, MBBS, Clinical Research Fellow at the University of Manchester
Co-Authors: S.A. Ali 1, A. Hoyle 2, N. James 3, C. Parker 4, C. Brawley 5, G. Attard 6, H. Douis 7, M. Mason 8, M. Parmar 5, M. Sydes 5, N. Clarke 2
2. The Christie and Salford Royal Hospitals, Manchester, UK
3. Queen Elizabeth-University Hospital Birmingham NHS Foundation Trust, Birmingham, UK
4. The Institute of Cancer Research/Royal Marsden NHS Foundation Trust, Sutton, UK
5. MRC Clinical Trials Unit at UCL, London, UK
6. University College London Cancer Institute, London, UK
7. University Hospital Birmingham, Birmingham, UK
8. Velindre Cancer Centre Velindre Hospital, Cardiff, UK
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain
Reference:
Further Related Content:


