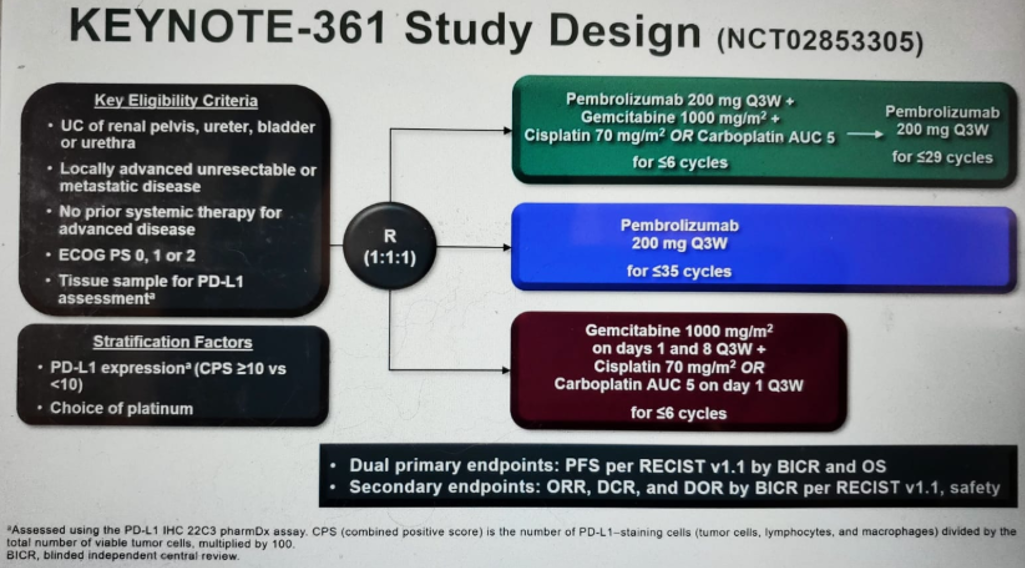
KEYNOTE-361 (figure 1) is a global, randomized, open-label, phase 3 trial of pembrolizumab alone or combined with platinum-based chemotherapy vs. chemotherapy as the first-line treatment for patients with advanced UC.
Figure 1 – KEYNOTE-361 study design:
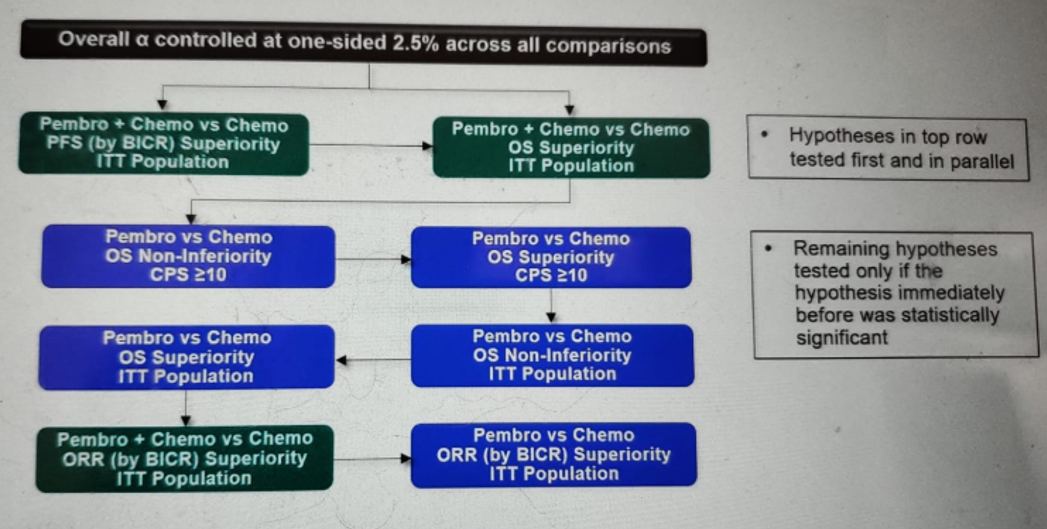
An important statistical consideration worth mentioning is that at the final analysis, alpha was adjusted for alpha spent at interim analysis to maintain an overall alpha=2.5% (one-sided) with an initial alpha of 0.5% and 2.0% allocated for progression-free survival (PFS) and overall survival (OS), respectively. Therefore, the p-value of statistical significance required for PFS and OS was 0.0019 and 0.0142, respectively. The analyses were performed in such a way that the hypothesis in the top row was tested first and in parallel (Figure 2), and the remaining hypotheses tested only if the hypothesis immediately before was statistically significant.
Figure 2 – Statistical considerations:
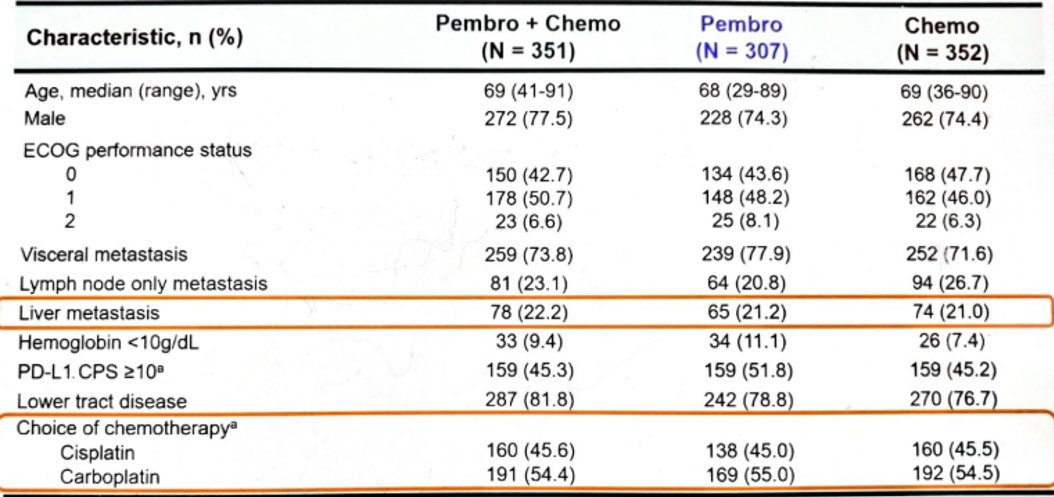
Patient disposition and baseline characteristics are shown in Figures 3 and 4, respectively.
Figure 3 – Patient disposition:
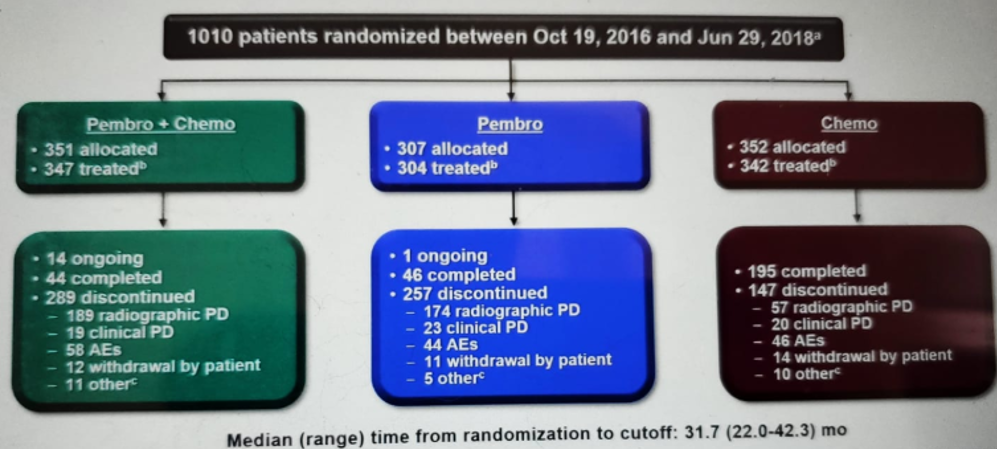
Figure 4 – Baseline characteristics:
The results of the first analysis comparing PFS in patients treated with pembrolizumab + chemotherapy vs. those treated with chemotherapy alone in the intention to treat (ITT) population demonstrated an advantage in favor of pembrolizumab + chemotherapy, but this did not reach the required statistical significance (Figure 5). The PFS by subgroups is shown in Figure 6.
Figure 5 – PFS in the ITT population:
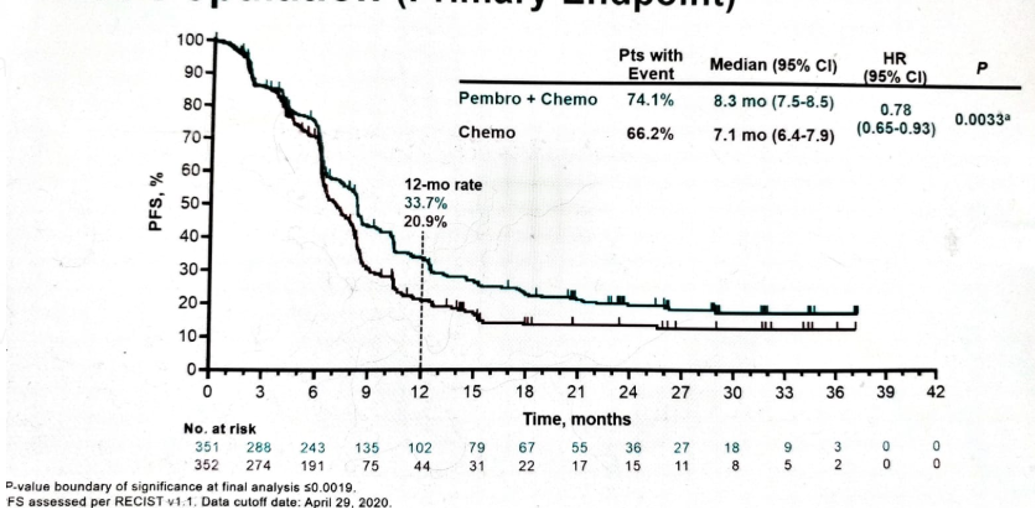
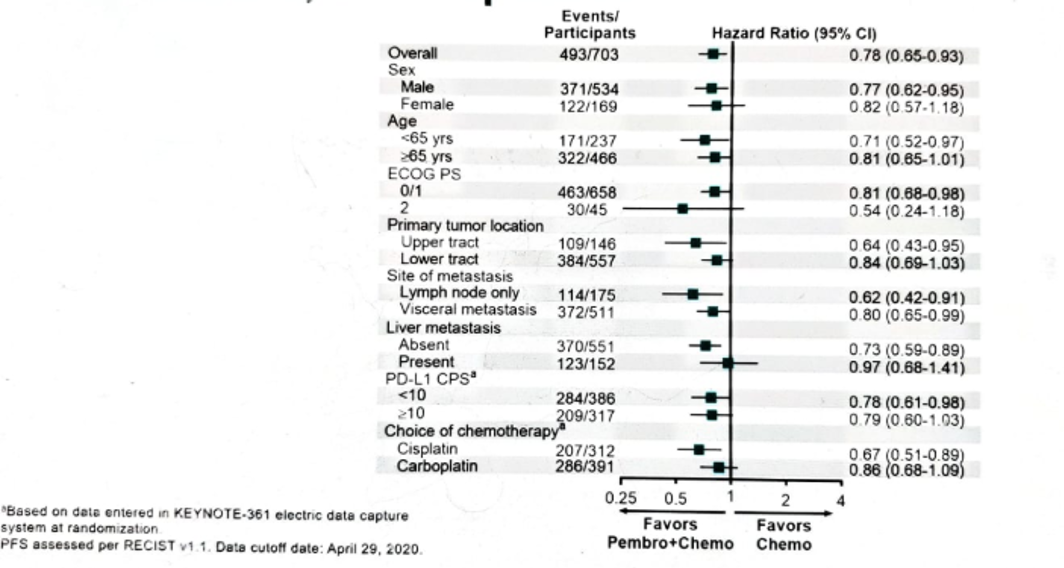
Figure 6 – PFS in subgroups:
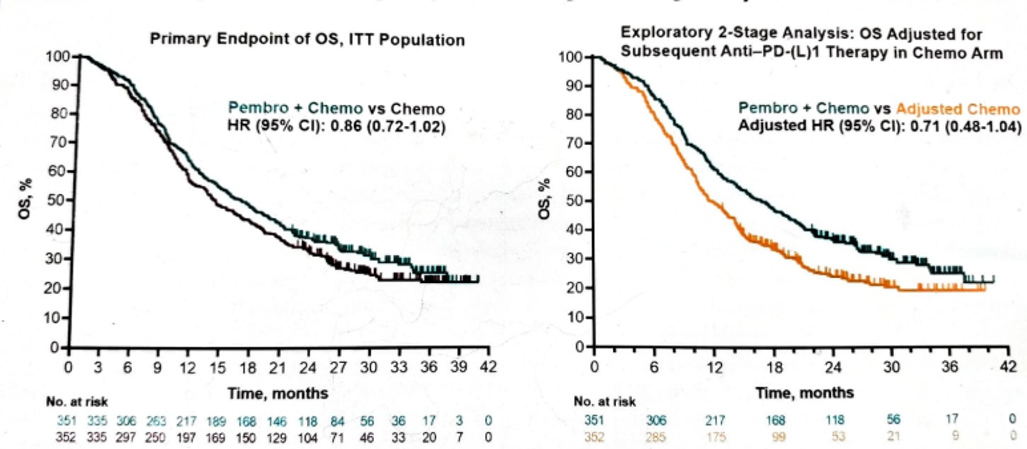
When assessing the OS, again, there was an advantage to pembrolizumab + chemotherapy, but this did not reach the required statistical significance level (Figure 7).
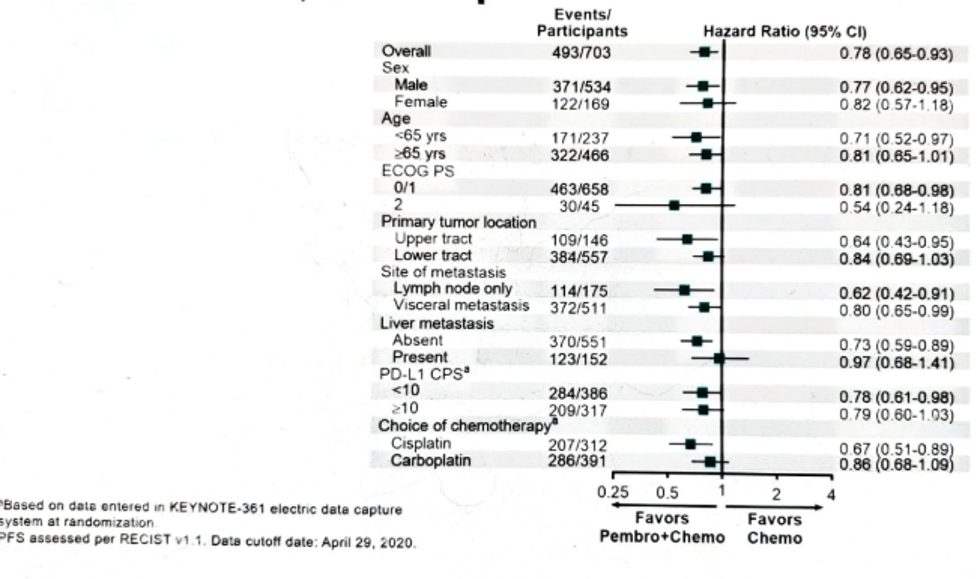
Figure 8 shows the OS by subgroups in the ITT population.
Figure 7 - Overall survival in the ITT population:
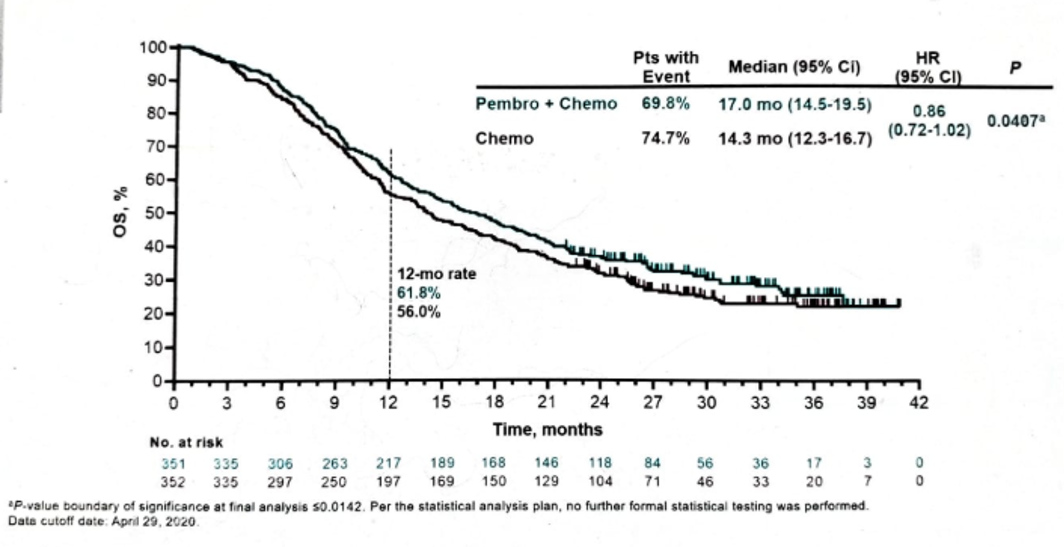
Figure 8 – Overall survival by subgroups:
Subsequent therapy in all treatment arms is shown in figure 9. In an exploratory analysis of OS, the effect of subsequent anti-PD-L1 therapy in the ITT population is shown in figure 10.
Figure 9 – Subsequent therapies in all treatment arms (ITT population):
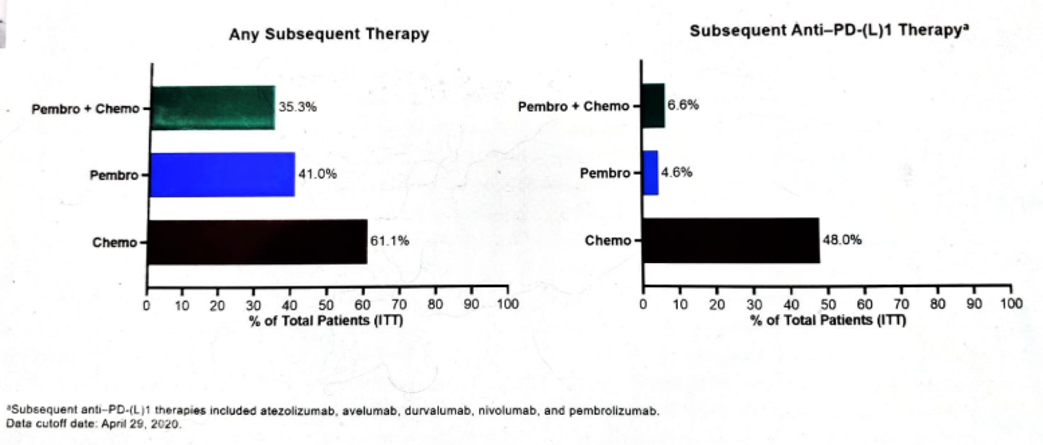
Figure 10 – Effect of subsequent Anti-PD-L1 therapy in the ITT population (exploratory analysis):
The all-cause adverse effects in the as-treated population are shown in figure 11, showing no significant addition of adverse events as a result of adding pembrolizumab to chemotherapy.
Figure 11- Adverse events in the as-treated population:
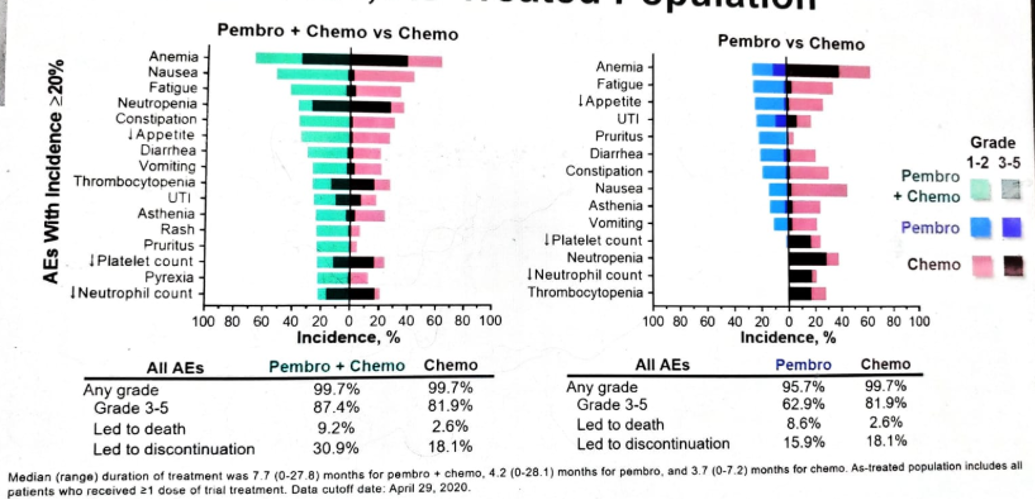
Summarizing his presentation, Dr. Ajjai stated that PFS and OS benefits of pembrolizumab + chemotherapy vs. chemotherapy alone did not reach the required statistical significance in patients with previously untreated advanced UC. Although formal statistical testing was not performed for the first-line pembrolizumab vs. chemotherapy in the ITT population, pembrolizumab did not appear to be superior to chemotherapy. However, pembrolizumab was associated with a more durable response. Lastly, the safety profile of pembrolizumab + chemotherapy was similar to that of chemotherapy. The safety profile of pembrolizumab was in line with prior studies, with a lower rate of all-cause adverse events compared to chemotherapy.
Presented by: Ajjai Alva, MD, MBBS, Medical oncologist at the University of Michigan, Ann Arbor, Michigan, United States
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020


