
As reported at the European Society of Medical Oncology (ESMO) 2019 meeting, this was the first randomized trial to show a progression-free survival benefit for immunotherapy in combination with chemotherapy. Final overall survival (OS) data from this trial have not yet been reported. Though the addition of atezolizumab did not add a major safety single to chemotherapy as measured by CTCAE adverse event reporting, these metrics do not reflect how treatment is experienced by patients or how treatment impacts their quality of life.
To assess patient-reported outcomes (PROs), the investigators utilized the EORTC QLQ-C30 scales of global health, function, and cancer-related symptoms, together described below. Questionnaires were administered at Day 1 of each treatment cycle, within 30 days of treatment discontinuation, and every three months after treatment discontinuation.
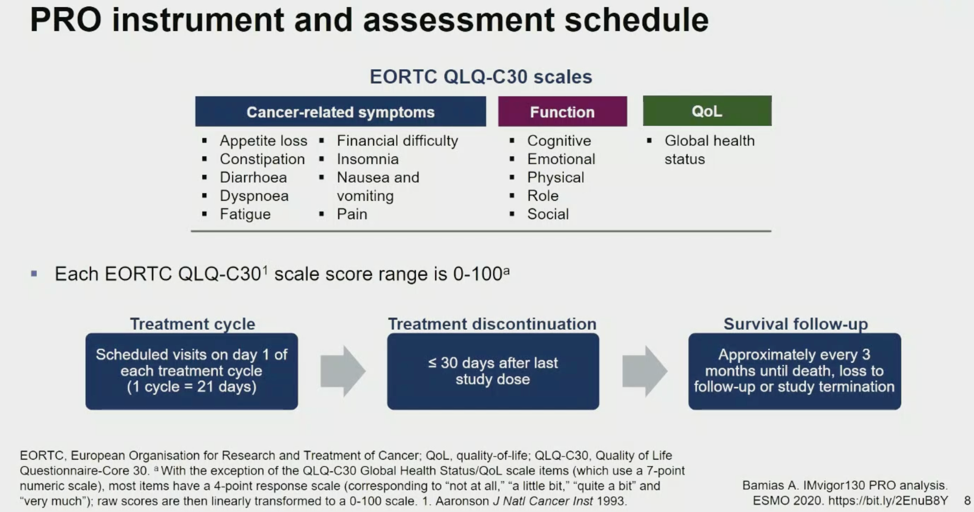
All analyses were pre-specified and secondary of exploratory. Results were described using the metrics of time to deterioration, defined as the time from randomization to the first >= 10-point score change above baseline, and change from baseline summaries, defined as a >= 10-point metric change with a treatment arm.
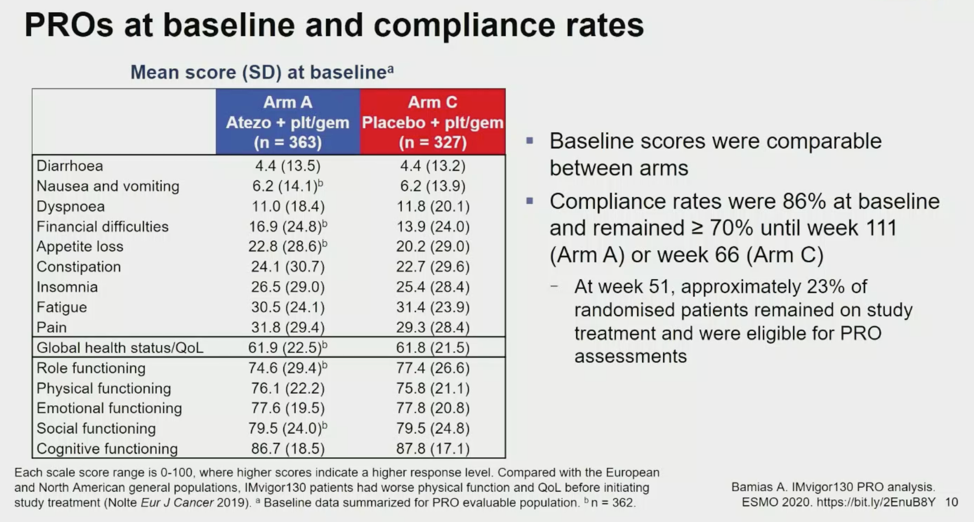
Results from each QLQ-C30 scale are shown below between treatment arms.
The was a trend towards a longer time to deterioration with combination immunotherapy/chemotherapy across almost all scale metrics, though the confidence interval for all hazard ratios crossed 1.

With regards to change from baseline in symptoms, there was a suggestion of improvement in emotional function with combination therapy relative to chemotherapy. In general, the addition of atezolizumab to chemotherapy did not compromise the patient-reported outcomes measured in this study. This will be important data to integrate with the already presented PFS benefit and forthcoming overall survival data when mature to weigh the overall benefit of combining atezolizumab to chemotherapy as first-line therapy in platinum-eligible patients with advanced urothelial carcinoma.
Presented by: Aristotelis Bamias, MD, National & Kapodistrian University of Athens, Athens, Greece
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020.
Related Content:
ESMO 2020: Combination Therapies in Locally Advanced or Metastatic Urothelial Carcinoma Bladder Cancer - Josh Meeks


