Of the 455 patients with mUC receiving ICB as any line therapy in the database, 71 had pre-existing autoimmune disease. These patients were older, had a higher Charlson Comorbidity Index without cancer, and more often had Medicare insurance. Most patients receive nivolumab as ICB (37.8%), followed by pembrolizumab (31%). Type 1 diabetes was the most common type of autoimmune disease (25.4%), followed by pernicious anemia (9.9%) and rheumatoid arthritis (9.9%). These data are summarized below.

In this cohort, the median unadjusted time to treatment discontinuation was numerically shorter than for patients with pre-existing autoimmune disease relative to patients without autoimmune disease (8.1 versus 6.8 months, p = 0.27). The authors used sensitivity analyses to assess outcomes in patients receiving ICB as first line therapy only, and the results were consistent with the main model of patients receiving ICB for any line therapy.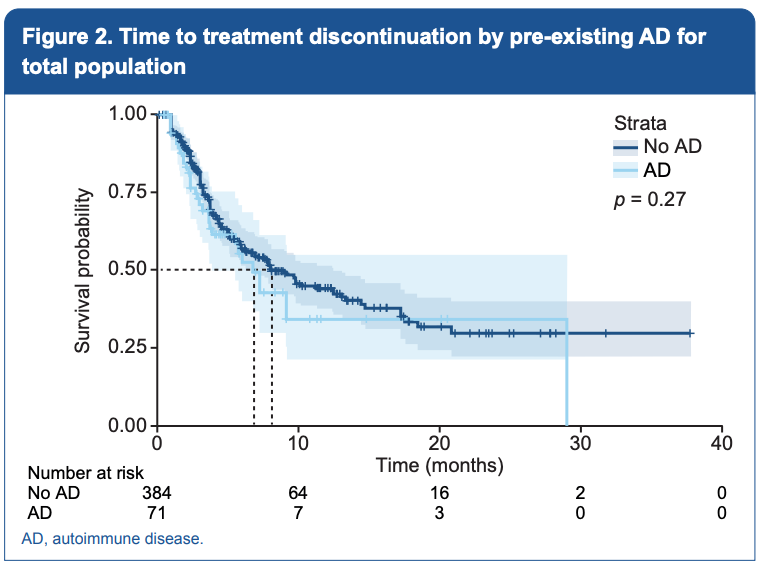
This study limited by the possibility of diagnosis miscoding and the possibility that a medical code was utilized as part of a rule-out workup. Studies utilizing larger cohorts of patients with pre-existing autoimmune conditions and ICB treatment are warranted to understand the safety and efficacy of this important treatment modality for these patients.
Presented by: Jean Hoffman-Censits, MD, Medical Oncologist, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins, Baltimore, Maryland, US


