(UroToday.com) Von Hippel-Lind (VHL) syndrome occurs because of hereditary inactivating genomic alterations in the VHL gene, resulting in a higher risk of developing renal cell carcinoma, retinal and CNS hemangioblastomas, and other tumors. Management of VHL syndrome-associated kidney neoplasms is challenging as tumors continue to arise despite surgical removal, which can result in renal insufficiency. To balance the risk of metastasis with renal morbidity, tumors are often observed until they reach 3 cm in size, at which point they are resected. Additional management strategies are required to manage VHL associated RCC.
Inactivation of VHL has been shown to result in aberrant oncogenic signaling through hypoxia-inducible transcription factors. MK-6482 is a potent and selective inhibitor of HIF-2a that has shown clinical activity in clear cell renal cell carcinoma. In this presentation, Dr. Ramaprasad Srinivasan reported the current phase 2 clinical outcomes data of MK-6482 in VHL disease-associated RCC. The schema of the trial is shown below.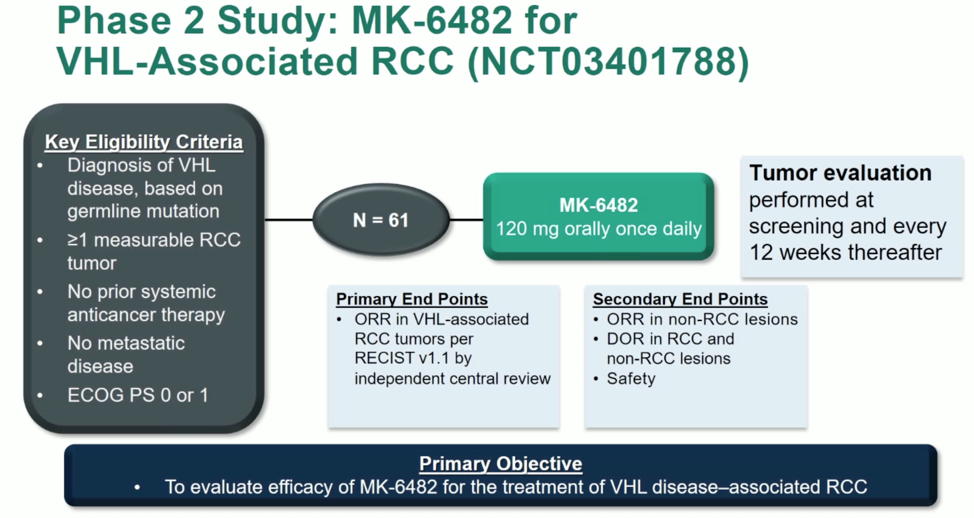
The primary outcome is ORR in non-metastatic disease, with tumor evaluation performed at screening and every 12 weeks. The baseline characteristics of the patients in this trial are notable for the younger age, consistent with VHL-associated malignancy, as well as the presence of other VHL-associated lesions.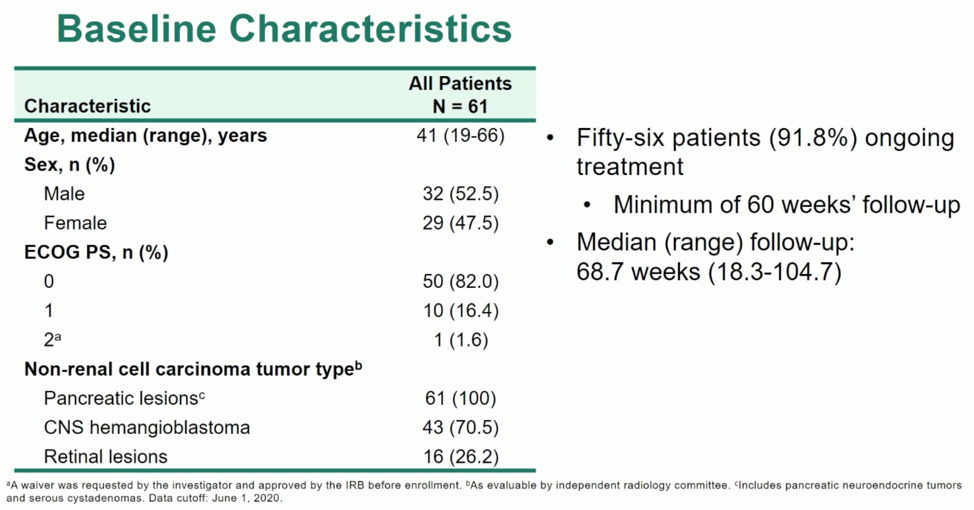
92% of patients had a decrease in the size of their target lesion, with an ORR of 36.1%. At a median follow-up of 68.7 weeks, responses appear to be durable, with 98% of patients free of progression at 52 weeks.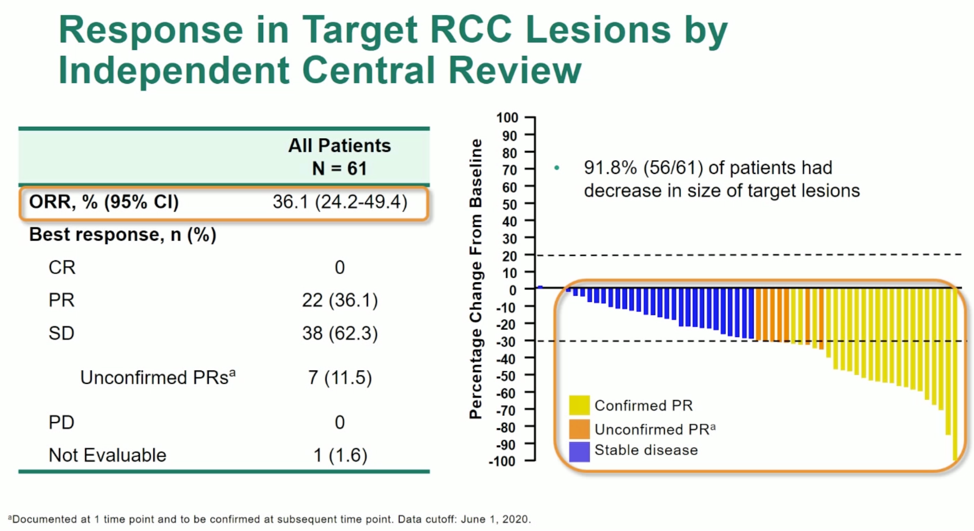
Notably, responses were also seen in non-RCC lesions associated with VHL disease.
Of 16 patients with retinal lesions, 11 patients had improvement in their retinal disease.
Almost all patients reported treatment-related adverse events. The most common grade 3+ toxicities were anemia, fatigue, and dyspnea.
In summary, MK-6482 showed promising clinical activity in VHL-disease-associated RCC as well as other associated lesions.
Presented by: Ramaprasad Srinivasan, MD, PhD, Principal Investigator and Head of the Molecular Therapeutics Section, Urologic Oncology Branch, National Cancer Institute, Bethesda, MD
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.


