Pazopanib has been approved for the treatment of advanced and/or metastatic RCC.2 Immune checkpoint inhibitors (ICIs) have been increasingly used in the front-line setting of metastatic clear-cell renal cell carcinoma (ccRCC).3,4 ICI and ICI-VEGF combinations are being adopted as treatment options for patients with metastatic RCC; however, vascular endothelial growth factor (VEGF) monotherapies may still play a major role in the management of this patient population.
To date, prospective data regarding the efficacy of pazopanib, specifically after previous treatment with an ICI are lacking. The authors of this study aimed to evaluate the safety and efficacy of pazopanib in patients with advanced or metastatic ccRCC following previous treatment with an ICI.
This was an international, single-arm, phase 2 trial (NCT03200717) (Figure 1)
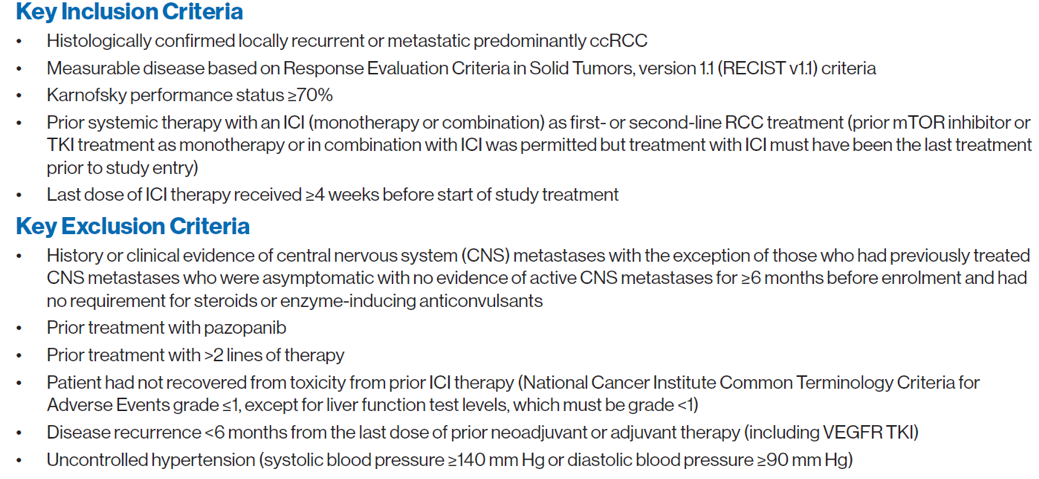
Pazopanib was given as a standard oral dose of 800 mg/day in treatment cycles of 28 days. Patients could continue on study treatment until two years after the last patient was enrolled. The presented poster shows the results of the primary analysis with a minimum follow-up of 6 months after the last patient was enrolled. The study is anticipated to be completed in mid-2021, with a final efficacy and safety follow-up analysis conducted at completion. The key inclusion and exclusion criteria are shown in figure 2. The primary efficacy endpoint was progression-free survival (PFS) based on local investigator assessment using RECIST v1.1. Secondary endpoints included objective response rate (ORR), overall survival (OS), Safety, and tolerability.
Figure 1 – Trial design:
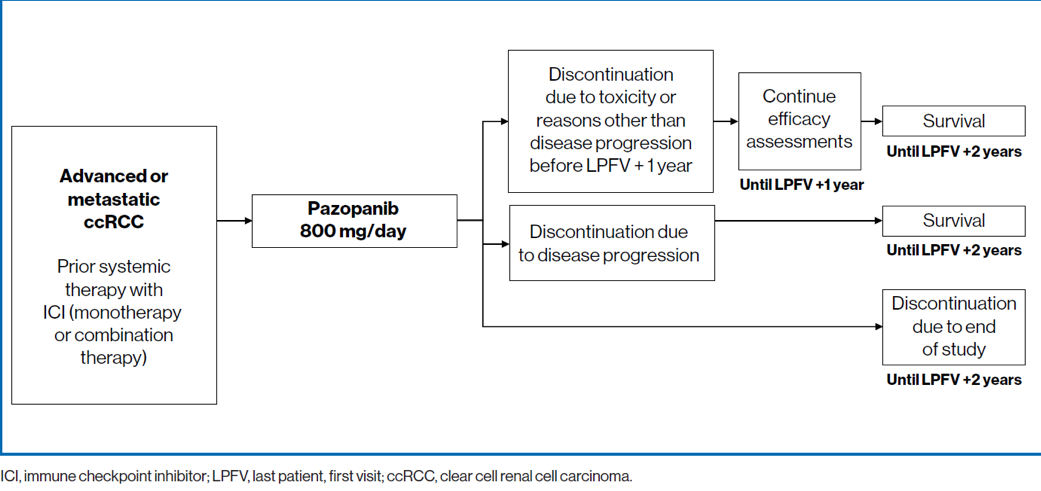
Figure 2 -Key inclusion and exclusion criteria:
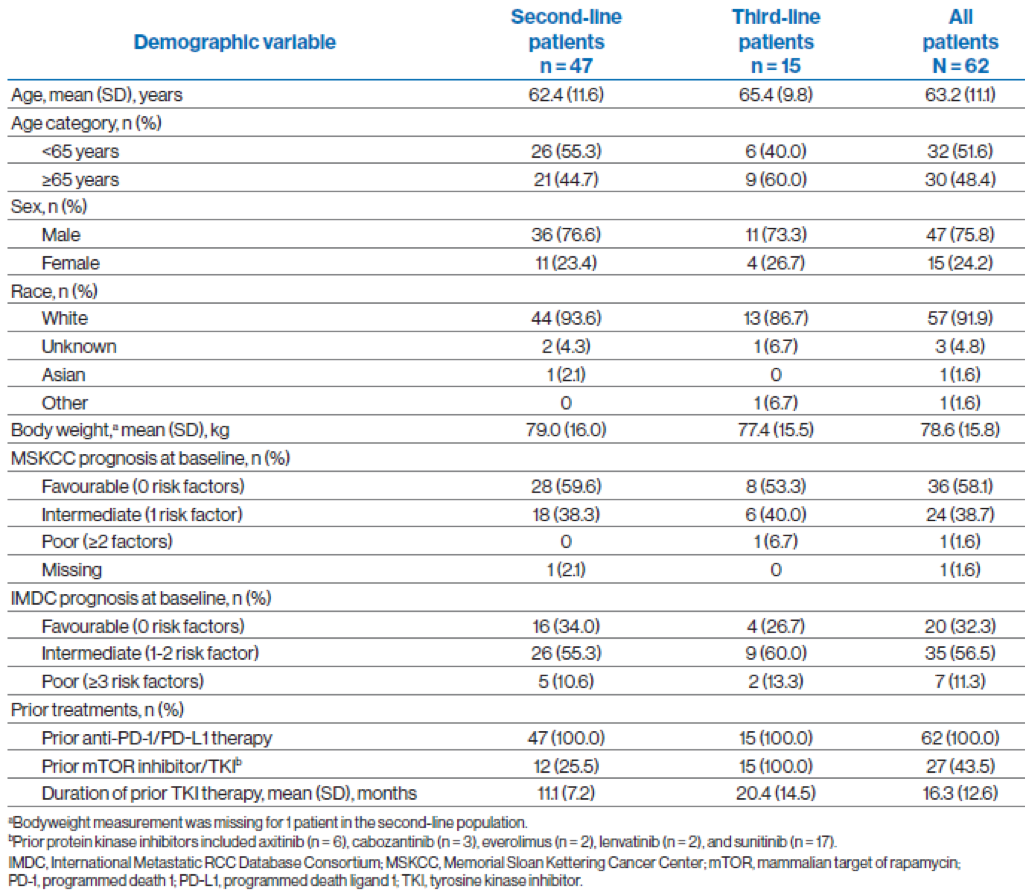
Key baseline characteristics are demonstrated in Table 1. At the data cutoff, 49 of 62 patients (79%) had completed treatment, and 13 patients (21%), all in the second-line treatment group, were still on treatment.
Table 1- Key demographics and baseline disease characteristics:
The results showed that the median PFS was 6.5 months (95% CI, 3.7-9.2 months) in all patients and 7.4 months (95% CI, 3.7-13.8 months) in the subgroup of patients treated with second-line pazopanib (Table 2 and Figure 2). The median PFS was similar in patients aged <65 years (5.7 months; 95% CI, 3.6-11.3 months) and those aged ≥65 years (6.5 months;95% CI, 3.7-9.2 months). The number of disease progressions was similar in patients aged <65 years (17/32, 53.1%) and those aged ≥65 years (18/30,60.0%) for the global population. The overall median OS was 21.3 months (95% CI, 14.9 months-not evaluable) (Figure 3). The median OS was 21.3 months (95% CI, 9.2-21.3 months) in patients receiving third-line pazopanib and has not been reached in those receiving second-line pazopanib (95% CI, 10.9 months-not evaluable).
The 12-month survival probability estimates were 68.2% (95% CI, 53.4-79.2%) in all patients, 65.8% (95% CI, 48.5-78.4%) in patients receiving second-line pazopanib, and 75.8% (95% CI, 40.4-91.9%) in those receiving third line pazopanib.
Table 2 – PFS:

In total, 9 of 47 patients receiving second-line pazopanib achieved an objective response (Table 4 and Figure 4). There were no complete or partial responses among patients receiving third-line pazopanib. The clinical benefit rate was 51.1% (95% CI, 36.1%-65.9%) for patients receiving second-line pazopanib and 40% (95% CI,16.3%-67.7%) for patients receiving third-line pazopanib. The adverse events are shown in table 6.
Figure 3 – PFS and OS in the full analysis:
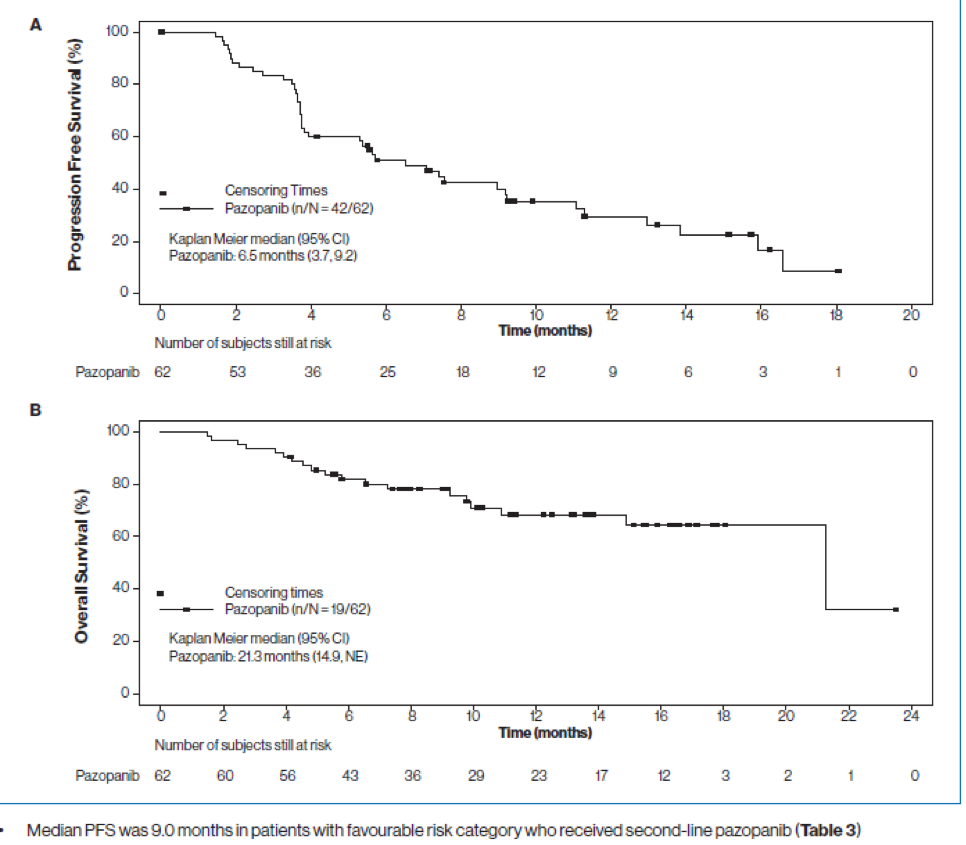
Table 3 – PFS in patients receiving 2nd line pazopanib with favorable risk:

Table 4 – Overall response rates:
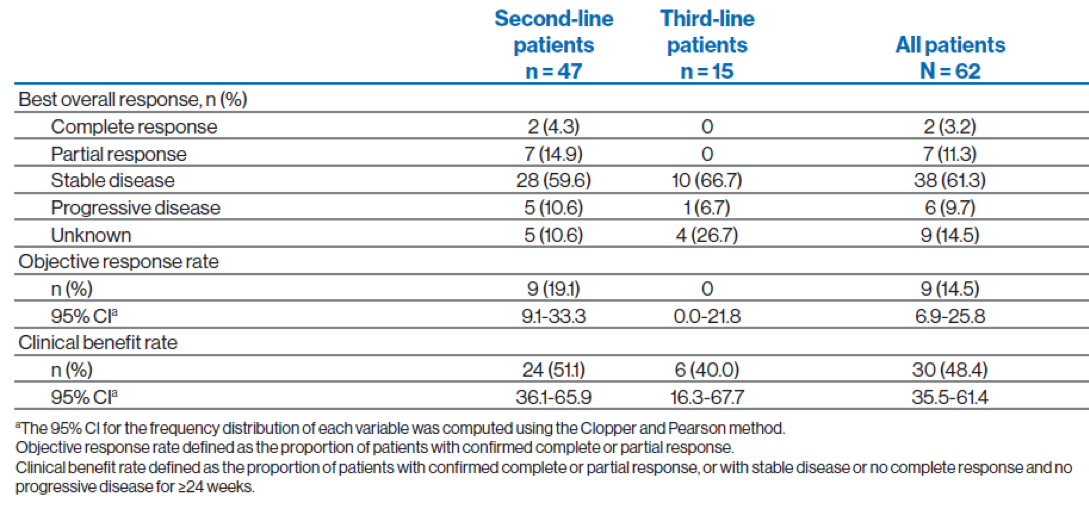
Figure 4 - Change from baseline in the sum of longest diameters based on local radiology review (all patients):
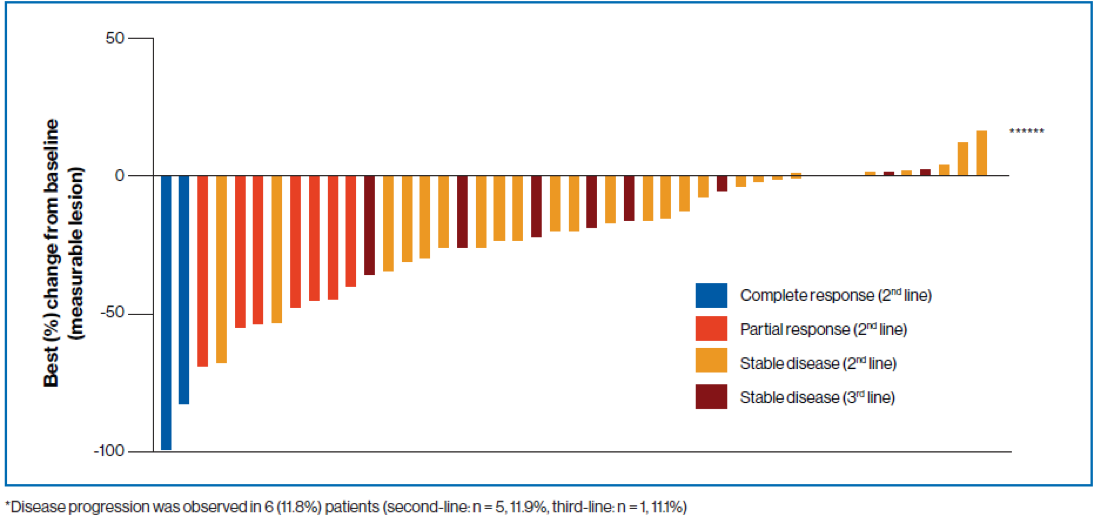
Table 6 – Adverse events:

In conclusion, a median PFS of 6.5 months (95% CI, 3.7-9.2 months) was achieved in patients with locally advanced or metastatic ccRCC receiving pazopanib after prior ICI treatment. A clinically meaningful median PFS of 9.0 months was achieved in favorable-risk patients who received pazopanib as second-line treatment. The median OS for all patients was 21.3 months (95% CI, 14.9 months-not evaluable) and has not been reached among those receiving second-line pazopanib. The authors state that treatment sequencing is important in the management of advanced or metastatic ccRCC, and patients may benefit from pazopanib treatment after progression following ICI therapy. The safety profile of pazopanib was similar to previous observations.
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Lead for Solid Tumour Research at Barts Cancer Institute, Director of Barts Cancer Institute, London, United Kingdom
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
References:
- Ward JE, Stadler WM. Pazopanib in Renal Cell Carcinoma. Clinical Cancer Research 2010; 16(24): 5923-7.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine 2013; 369(8): 722-31.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2019; 380(12): 1116-27.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018; 378(14): 1277-90.


