(UroToday.com) Targeted therapy has significantly changed the treatment landscape for renal cell carcinoma, and more recently, immuno-oncology–based combinations have also made a significant impact. Treatment choice in advanced renal cell carcinoma (aRCC) is informed by patient risk, as measured by the validated International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk model. This model categorizes patients into favorable (F), intermediate (I), and poor (P) risk groups according to prognosis1.
Overall survival (OS) benefits in first-line treatment of aRCC for patients in I/P IMDC risk groups have been demonstrated in randomized clinical trials (RCTs) comparing nivolumab plus ipilimumab (Nivo+IPI) (CheckMate 214), pembrolizumab plus axitinib (KEYNOTE-426), or Cabozantinib (CABOSUN) with sunitinib2-4:
— In CheckMate 214, a phase 3 RCT, Nivo+Ipi demonstrated statistically significant improvement in OS versus sunitinib (hazard ratio [HR], 0.63; 99.8% CI, 0.44-0.89), with a minimum follow-up of 17.5 months2. Recently, CheckMate 214 demonstrated continued OS benefit versus sunitinib with a minimum follow-up of 42 months (HR, 0.66; 95% CI, 0.55-0.80; P < 0.0001)5
— In KEYNOTE-426, a phase 3 randomized controlled trial comparing pembrolizumab+ axitinib versus sunitinib in first-line treatment of aRCC, benefit was demonstrated
in a subgroup analysis of intermediate and poor-risk patients with a minimum of approximately 11 months of follow-up (HR, 0.55; 95% CI, 0.41-0.74; P = 0.00004). The primary endpoint in the all-risk population was met3.
— In CABOSUN, a phase 2 randomized controlled trial, cabozantinib demonstrated an improved OS relative to sunitinib (HR, 0.80; 95% CI, 0.53–1.21) with a median follow-up of 35.4 months4
In this presented work, the authors’ objective was to evaluate the lifetime survival and cost-effectiveness of Nivo+Ipi compared with other systemic treatments (pembrolizumab+ axitinib, cabozantinib, and sunitinib) for first-line treatment of patients with intermediate/fall-risk aRCC in a US setting.
Evidence was included from CheckMate 214, KEYNOTE-426, and CABOSUN for progression-free survival [PFS] and overall survival (OS) with a median follow-up of 25 months for PFS analysis and 35.4 months for OS analysis.
PFS extrapolations provided a good fit to the trial data, including the apparent plateau in PFS for Nivo+Ipi after approximately two years (Figure 2A). PFS for Pembrolizumab + Axitinib was seen to drop below that of Nivo+Ipi from approximately 18 months and converges toward sunitinib (Figure 2A), which is also reflected in the extrapolated PFS over a 40-year time horizon (Figure 2B). Nivo+Ipi showed a benefit in terms of PFS compared with both sunitinib and pembrolizumab + axitinib over the 40-year time horizon of the model (Figure 2B).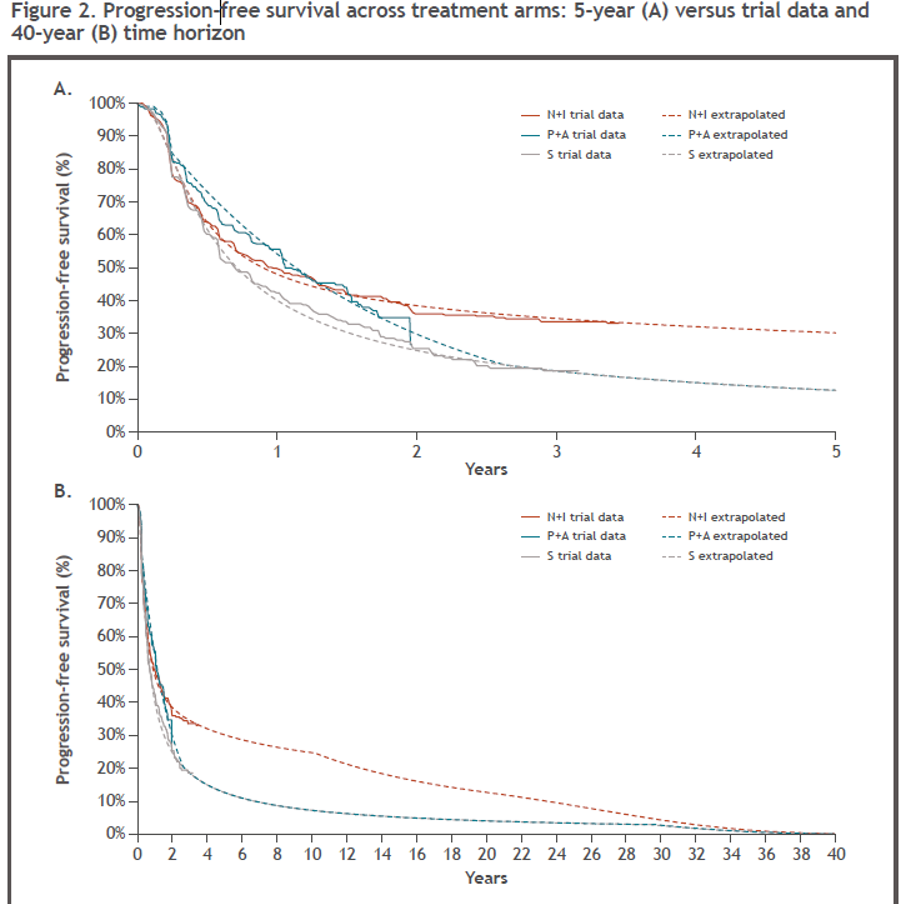
Landmark survival estimates were in line with reported data within the observed trial periods. OS estimates at 5 and 10 years were higher for Nivo+Ipi and for pembrolizumab + axitinib than for Sunitinib (Table 2). While extrapolations fit in-trial estimates well for Cabozantinib, the 5- and 10-year estimates relative to sunitinib estimates were deemed clinically implausibly low, due to the CABOSUN results. This could be due to differences in the availability of subsequent therapies given the timing of both trials.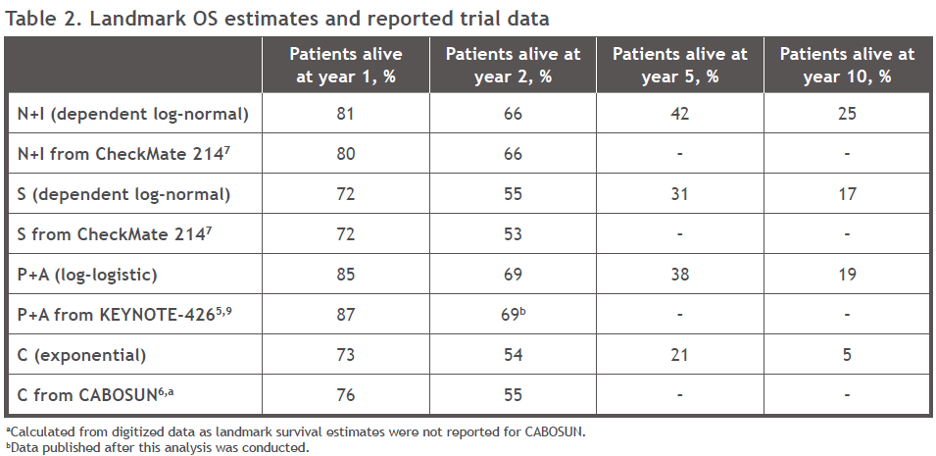
The five-year extrapolated OS curves were compared with Kaplan–Meier curves, reported in the relevant randomized controlled trial publications (Figure 3A). These extrapolations were in line with data reported from the trials. Over the 40-year model time horizon, the extrapolated OS curve for Nivo+Ipi showed a survival benefit over both sunitinib and pembrolizumab + axitinib, while OS for pembrolizumab + axitinib appeared to converge toward that of sunitinib after ten years (Figure 3B).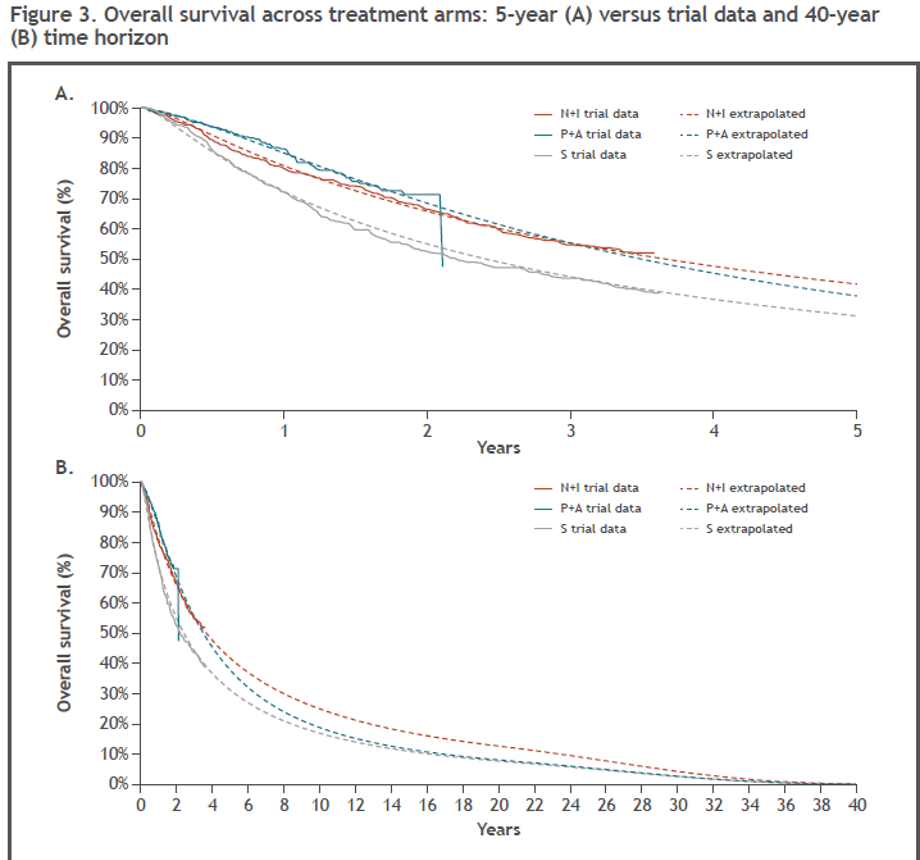
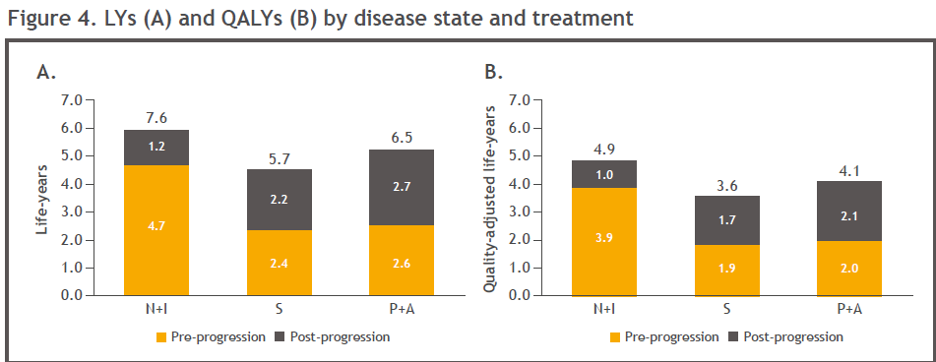
Nivo+Ipi treatment was associated with the highest total life years of all treatments (Figure 4A). Nivo+Ipi generated higher pre-progression life years compared with pembrolizumab + axitinib, due to the plateau seen in the Kaplan–Meier data for Nivo+IPi, which has not been demonstrated to date for pembrolizumab + axitinib. Compared with sunitinib, Nivo+IPi was associated with more than two times the life-years gain achieved with pembrolizumab + axitinib (5.7 life-years vs. 7.6 and 6.5 life-years, respectively).
Nivo+Ipi was also associated with the highest (4.9) mean quality-adjusted life year (QALYs) compared with pembrolizumab + axitinib (4.1) and sunitinib (3.6) (Figure 4B and Table 3). Nivo+Ipi yielded more than double the QALY gain versus sunitinib when compared with pembrolizumab + axitinib versus sunitinib (1.3 vs. 0.5, respectively).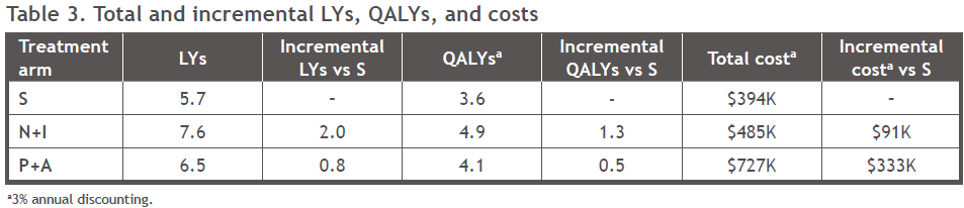
Pembrolizumab + axitinib was associated with the highest total cost ($727K), followed by Nivo+Ipi ($485K), and sunitinib was associated with the lowest total cost ($394K; Figure 5). The highest proportional cost was drug acquisition for pembrolizumab + axitinib and Nivo+Ipi, while the highest proportional cost for sunitinib was subsequent treatment (Figure 5). The percentage of total drug cost was 55.1% for Nivo + Ipi, 40.7% for sunitinib, and 70.6% for pembrolizumab + axitinib.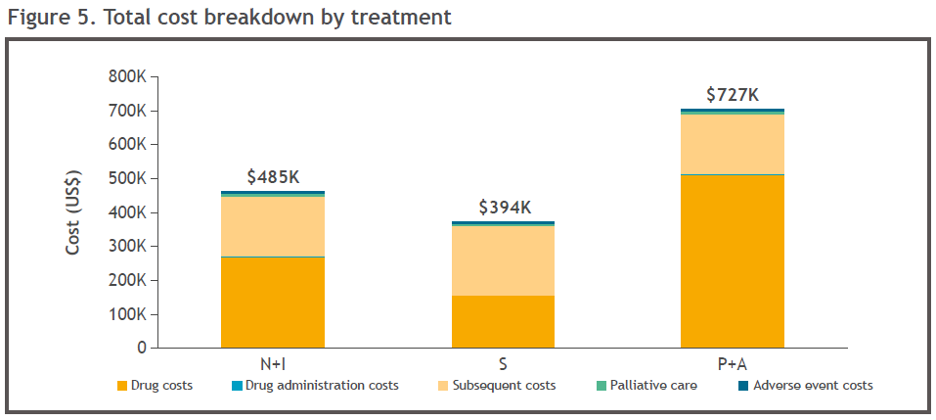
Some of the limitations of this study include the lack of ability to correct for heterogeneity between studies. Also, the follow-up for pembrolizuab+axitinib was limited at the time of analysis, and survival data from KEYNOTE426 were immature.
In conclusion, Nivo+Ipi offers aRCC patients with intermediate or poor-risk a potential cost-effective treatment option with higher estimated mean long-term survival versus other first-line treatments in the United States. Over a lifetime, Nivo+Ipi led to increased survival when compared to sunitinib and pembrolizumab + axitinib with lower total incremental costs, when compared to sunitinib and compared with pembrolizumab + axitinib treatment.
When putting it all together, these results suggest that Nivo+Ipi is a more effective first-line treatment than pembrolizumab + axitinib and sunitinib.
Presented by: Toni K. Choueiri, MD, Jerome and Nancy Kohlberg Professor of Medicine, Harvard Medical School, Attending Physician, Solid Tumor Oncology, Dana-Farber Cancer Institute, Director, Genitourinary (GU) Oncology Disease, Center, Dana-Farber Cancer Institute, Director, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA, @GoldbergHanan at the2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
References:
1. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. The Lancet Oncology 2013; 14(2): 141-8.
2. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018; 378(14): 1277-90.
3. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2019; 380(12): 1116-27.
4. Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. European journal of cancer (Oxford, England : 1990) 2018; 94: 115-25.
5. Motzer RJ, Escudier B, McDermott DF, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. Journal for immunotherapy of cancer 2020; 8(2).
Related Content:
ASCO 2020: Biomarker Analyses from the Phase III CheckMate 214 Trial of Nivolumab plus Ipilimumab or Sunitinib in Advanced Renal Cell Carcinoma
CABOSUN Trial Plus an In-depth Discussion of Kidney Cancer Treatments - Toni Choueiri
KEYNOTE-426: Pembrolizumab plus Axitinib versus Sunitinib as First-Line Therapy for Advanced Renal Cell Carcinoma (RCC) - Brian Rini


