This trial compared nivolumab and cabozantinib combination therapy versus sunitinib as first-line treatment for advanced clear cell renal cell carcinoma. The schema of the trial is shown below. All IMDC risk groups were enrolled. Cabozantinib was administered at a lower dose of 40 mg daily in this combination regimen. The primary endpoint was progression-free survival, with overall survival, response rate, and safety as key secondary endpoints. Exploratory analyses included quality of life metrics and pre-specified subgroup analyses.
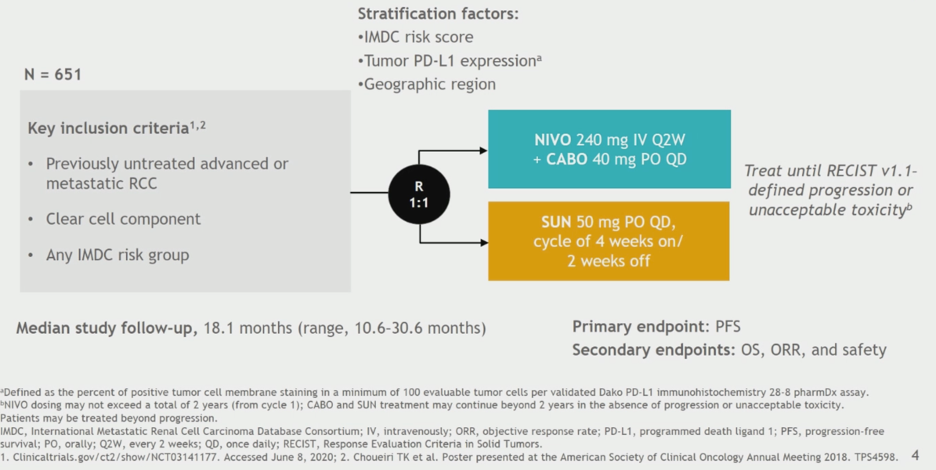
The data presented is based on a median study follow-up of 18.1 months with data locked on March 30, 2020. The statistical testing for the study was performed in a hierarchical manner as shown.
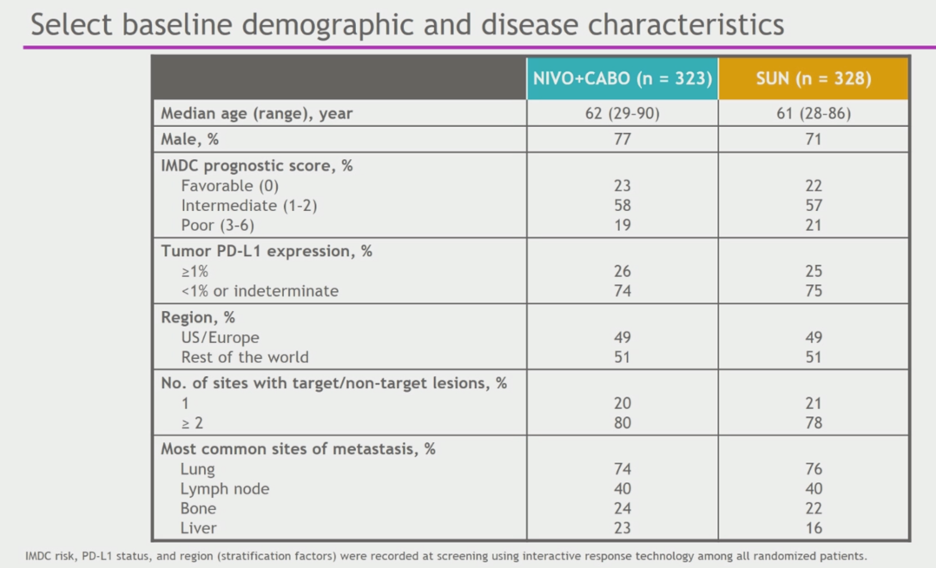
Patient characteristics are shown below. Patients were mostly male, IMDC intermediate risk, and PD-L1 negative/indeterminate.
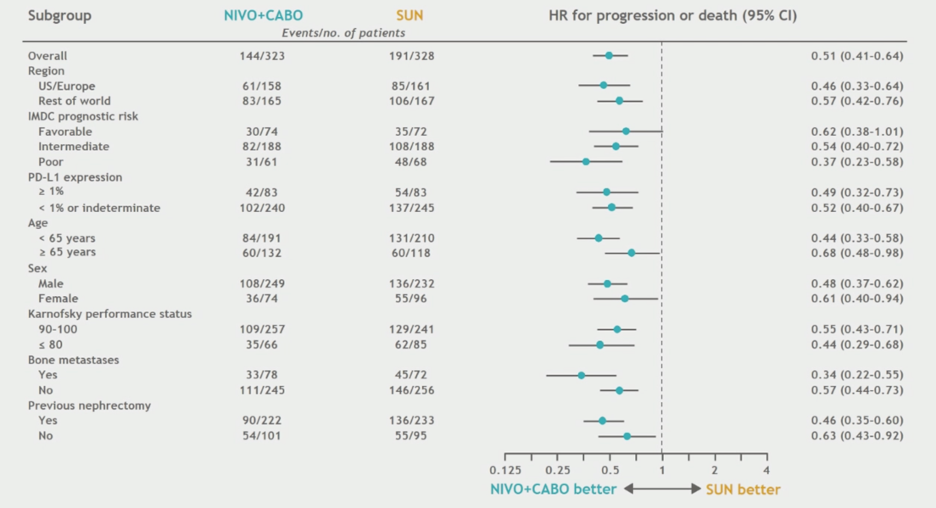
The trial met its primary endpoint, demonstrating superior progression-free survival for combination nivolumab and cabozantinib relative to sunitinib. Median PFS for the combination treatment was 16.6 months, and median PFS for sunitinib was 8.3 months. The hazard ratio for progression was 0.51 (0.41 – 0.64, p < 0.0001).

Pre-specified subgroup analyses were consistent with the observed overall trend of improved PFS with nivolumab + cabozantinib.
Overall survival analysis at the data cut-off demonstrated longer overall survival for the nivolumab and cabozantinib combination group. The median survival was not reached in either treatment group at this time point, and the hazard ratio for death with combination therapy was 0.60 (0.4 – 0.89, p = 0.001). Subgroup analyses were consistent with the overall finding of longer survival with combination therapy.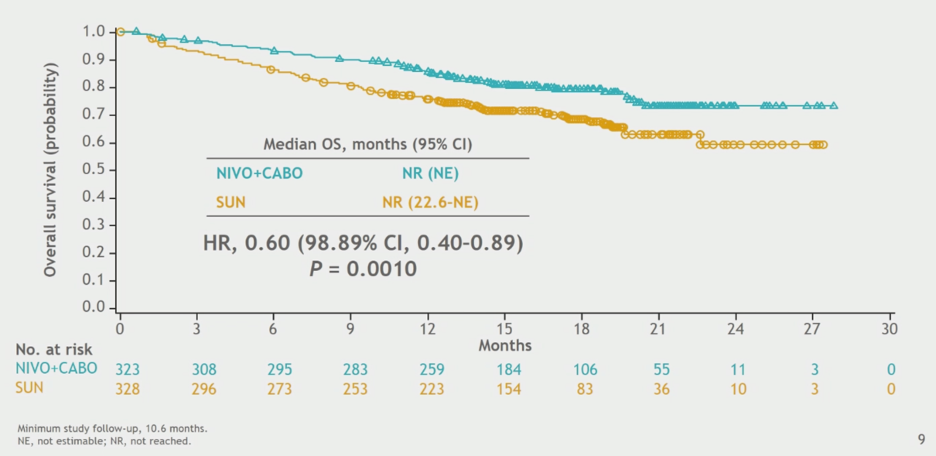
Combination nivolumab and cabozantinib resulted in a higher ORR of 55.7%, with 8% CR rate, and ORR was higher with combination regardless of IMDC risk group or PD-L1 expression. Median time to response and median duration of response were better with the combination.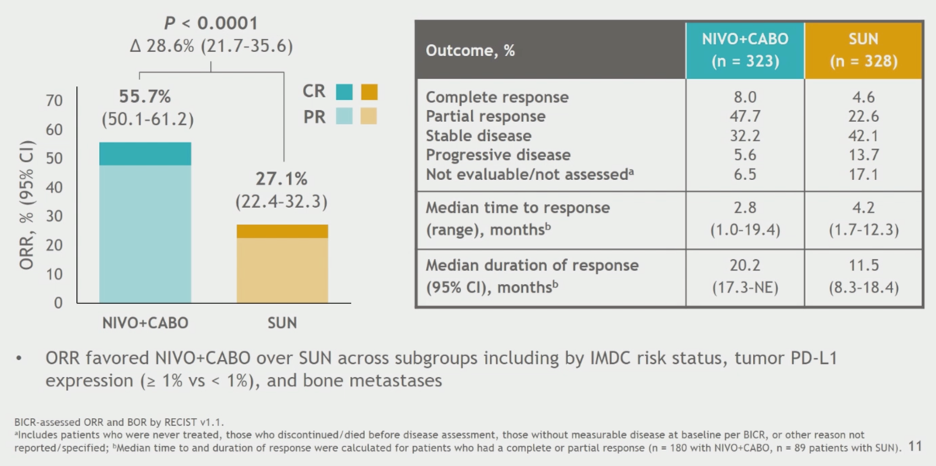
With regards to treatment tolerability, no dose reductions were allowed with nivolumab, but dose reductions in cabozantinib and sunitinib were permitted. A comparable number of patients required dose reductions in each arm. More patients had treatment of at least one drug discontinued in the combination arm (15.3% versus 8.8%) due to treatment-related adverse events.
Below is a tornado plot of adverse events noted. The rate of Grade 3+ treatment-related AEs was higher in the combination arm, more notably with regards to ALT elevation relative to sunitinib. There was one death attributed to combination therapy, and 2 deaths attributed to sunitinib therapy.
Immune-related adverse events are shown below.
Quality of life was assessed using the FKSI-19 total score and FKSI disease-related symptom subscale. Combination nivolumab and cabozantinib maintained FKSI-19 score throughout therapy as opposed to a decrease in quality of life seen with sunitinib. With regards to the disease-related symptom subscale, combination therapy improved scores over time, whereas sunitinib therapy was associated with deterioration.
Similar numbers of patients in each arm went on to receive subsequent therapy. 40% of patients in the sunitinib arm went on to receive other systemic therapy, and 29% of patients in this arm received PD-1 axis immunotherapy.
In summary, nivolumab and cabozantinib met its primary endpoint demonstrating higher PFS relative to sunitinib. Secondary endpoints of overall survival and ORR were also improved with the combination, as were patient report quality of life metrics. These data support nivolumab and cabozantinib combination as a potential first line therapy for advanced clear cell RCC, adding to other drug approvals in this space.
The choice for which treatment option to utilize for first-line therapy in patients with clear cell advanced RCC will likely depend on need for cytoreduction as balanced with safety profile, quality of life while on treatment, and cost.
Presented by: Toni K. Choueiri, MD, Director of the Lank Center for Genitourinary Oncology at the Dana-Farber Cancer Institute and Professor of Medicine, Harvard Medical School, Boston, Massachusetts
Related Content:
ASCO GU 2020: Comparing Nivolumab versus Everolimus with >5 years of Follow-up in Patients with Advanced Renal Cell Carcinoma: A Final Analysis of the CheckMate 025 Trial


