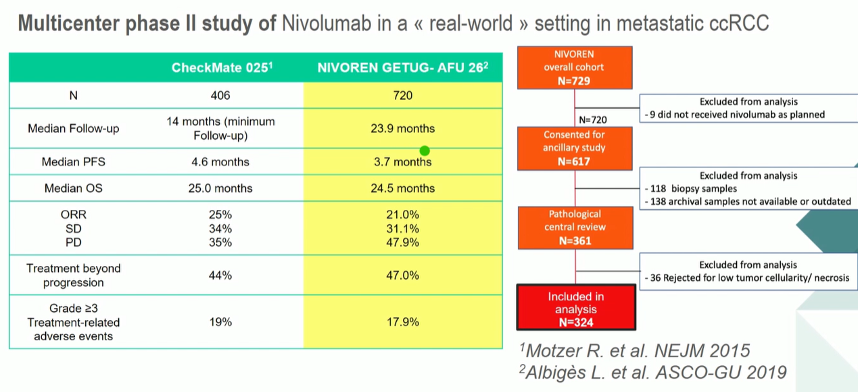
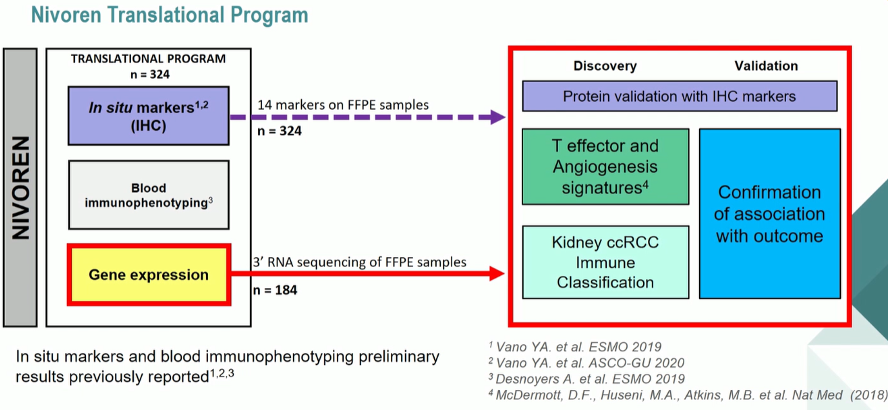
In conjunction with the clinical outcomes assessed in this study, a translational research program was initiated to identify biomarkers to predict outcomes from nivolumab treatment. In a proffered paper presentation at this year’s European Society of Medical Oncology (ESMO) 2020 Virtual Annual Meeting, Dr. Meylan presented the results of this analysis.
Among the 720 patients enrolled in NIVOREN, 324 patients were included in the NIVOREN translational cohort. RNA sequencing was performed on 79 primary clear cell renal carcinoma specimens. The authors first examined the effect of T-effector and Angiogenesis signatures on patient outcomes, based on median messenger RNA (mRNA) expression determined in the IMmotion150 trial. The authors then undertook unsupervised analysis to classify tumors according to infiltration by eight immune and two stromal cell population, in order to better understand the tumor microenvironment. These signatures were correlated with clinical outcomes including response rate (i.e. best observed response, partial or complete response) and progression-free survival.
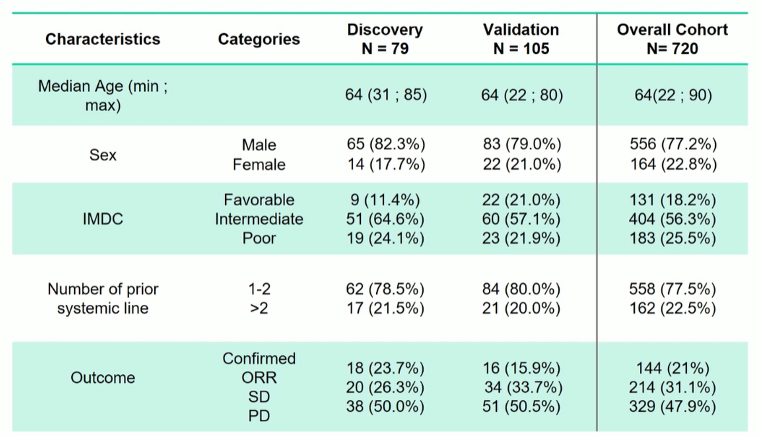
Included patients represent a relatively standard cohort for this disease space. Notably, those included in the Discovery and Validation cohorts were similar to each other and to the overall cohort.
The authors failed to demonstrate a predictive response of either the Angiogenesis or T-effector signatures on clinical outcomes when examined separately. However, when combined, there was a significant association for both response rate and progression-free survival, with better outcomes for patients with Teff-high/Angio-low and worse outcomes for those with Teff-low/Angio-low.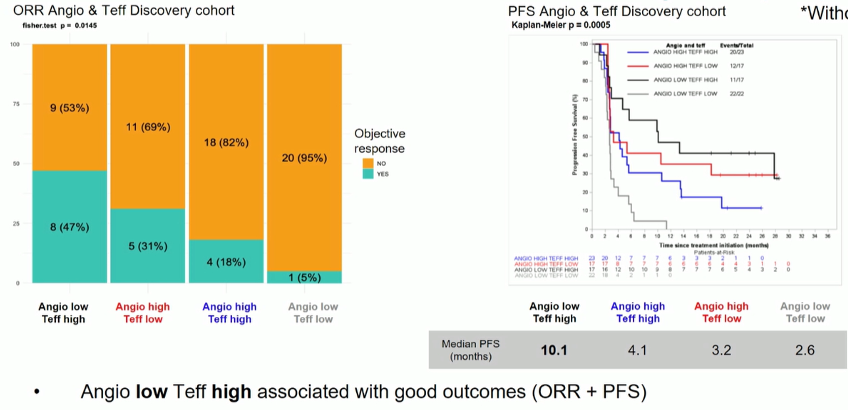
Unsupervised classification analysis led to the identification of five unique subtypes, which the authors labeled A through E.

The authors then sought to assess for overlap between their two classification strategies, with minimal overlap (30%).
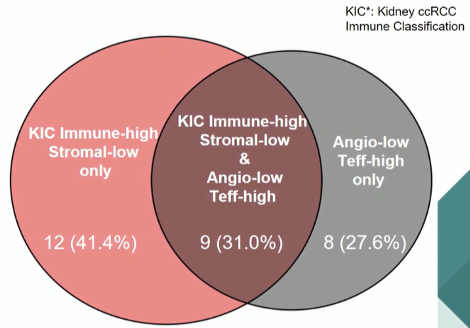
Higher response rates and longer progression-free survival was seen in patients with CD8-high/Stromal-low (KIC C and E categories) tumors than those with Immune-low/Stromal-low (KIC A), Immune-low/Stromal-high (KIC B), and Immune-high/Stromal-high (KIC D) tumors.
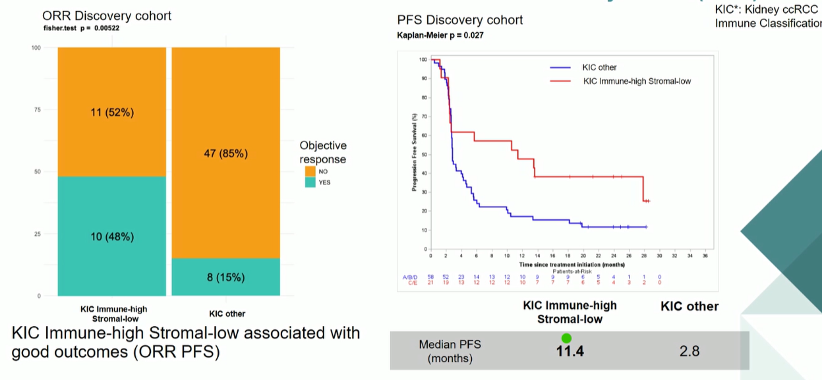
These results were confirmed in their validation cohort of a separate 105 patients.
The authors conclude that their signature can identify patients, with Immune-high/angiogenesis- and stromal-low signatures, who are most likely to receive maximal benefit from treatment with nivolumab for the treatment of metastatic renal cell carcinoma following initial therapy.
Presented by: Maxime Meylan, PhD Student at Cordelier Research Center Bioinformatics - Immunology - Cancer, Paris, France
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Contact: @WallisCJD on Twitter at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
Related Content:
ASCO GU 2020: Comparing Nivolumab versus Everolimus with >5 years of Follow-up in Patients with Advanced Renal Cell Carcinoma: A Final Analysis of the CheckMate 025 Trial


