For COSMIC-021, patients with clear cell RCC were enrolled in the dose-escalation (N=10) and expansion stage (N=60) of the study. Patients were enrolled sequentially to receive atezolizumab 1200 mg IV every three weeks with either cabozantinib 40 mg (dose level 40 [DL40], n=34), or cabozantinib 60 mg (DL60, n=36) PO daily in each stage. Eligible patients had to have an ECOG performance status of 0-1 and not have received prior systemic anticancer therapy for advanced RCC. The primary endpoint for this trial is objective response rate (ORR) per RECIST v1.1 by investigator, the secondary endpoint was safety, and exploratory endpoints include PFS and correlation of biomarkers with outcomes. The data presented at ESMO 2020 was for all 70 clear cell RCC patients with a data cutoff date of July 21, 2020. As follows is a summary of the trial design:
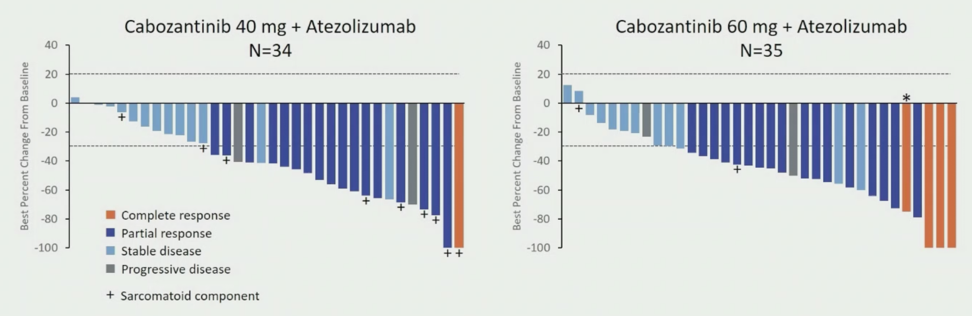
At the time of data cutoff, 70 patients with clear cell RCC (34 at DL40 and 36 at DL60) had a median follow-up of 25.8 months (range: 20-33) for DL40 and 15.3 (range: 10-32) for DL60. Baseline characteristics were similar in the two dose groups. The median age for all patients was 65 years of age, 76% were male, 74% had a ECOG performance status of 0, 87% had a prior nephrectomy, 77% had lung metastases, and 46% had ≥3 sites of disease. With regards to IMDC risk status, 30% were favorable, 67% were intermediate, and 3% were poor risk. For DL40, the ORR was 53% (80% CI 41-65), with one complete response (3%) and 17 partial responses (50%), the disease control rate was 94%, duration of response was not reached (range: 12.4 months to not reached), and the median time to objective response was 1.4 months (range: 1-19). For DL60, the ORR was 58% (80% CI 46-70), with four complete responses (11%) and 17 partial responses (47%), the disease control rate was 92%, the median duration of response was 15.4 months (range: 8.1 to not reached), and median time to objective response was 1.5 months (range: 1-7). The best change from baseline in sum of the target lesions is as follows, including those with a sarcomatoid component:
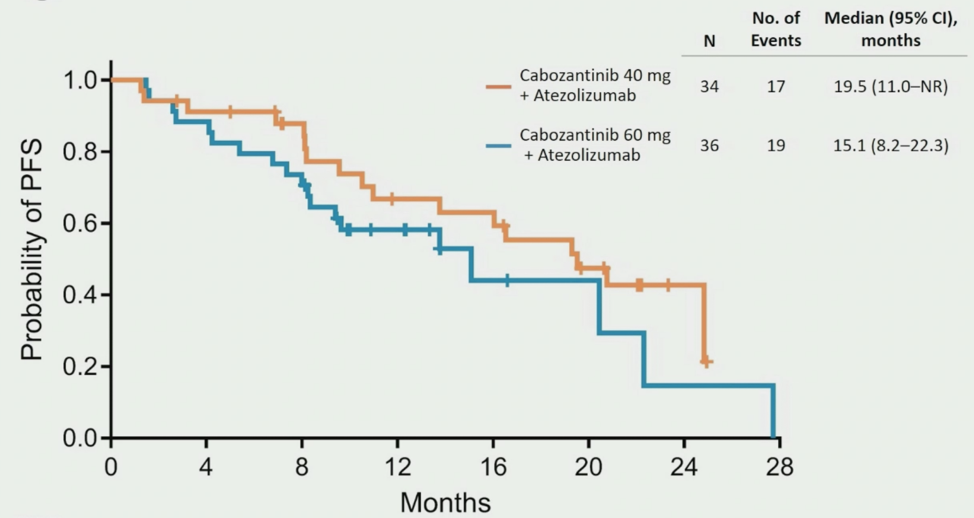
For DL40, the median PFS was 19.5 months (95% CI 11.0 to not reached) compared to 15.1 months (95% CI 8.2-22.3) for DL60:
Available tumor tissue (n=40) was evaluated for PD-L1 expression, and no association with antitumor activity was shown. Increased median levels of activated peripheral cytotoxic T (+8%) and NK (+24%) cells were observed at day 21 with a concomitant decrease in immunosuppressive cells. Baseline PD-L1+ and higher levels of CD8+ T cells was associated with greater tumor lesion reduction and was significantly associated with overall response:

The best overall response per RECIST v1.1 by tumor infiltration lymphocyte immune phenotype included 33% for immune-low (4/12), 47% for myeloid dominant (8/17), and 77% for T-cell rich (10/13). At the time of data cut-off, 41% of DL40 patients were still on treatment, compared to 50% of DL60 patients. For DL40 patients, 21% discontinued treatment due to radiographic progression, compared to 31% for DL60 patients. Grade 3/4 treatment-related adverse events occurred in 71% of DL40 and 64% of DL60 patients, with no grade 5 events at either dose. The most common grade 3/4 treatment-related adverse events in all patients were hypertension (24% in DL40 and 14% in DL60), diarrhea (9% and 19%), hypophosphatemia (15% and 3%), and ALT increased (3% and 14%). Three (9%) DL40 patients and 9 (25%) DL60 patients required high-dose steroids for immune-related adverse events.
Dr. Pal concluded his presentation of the initial results of the COSMIC-021 phase Ib trial with the following concluding statements:
- The combination of cabozantinib plus atezolizumab demonstrated encouraging clinical activity in previously untreated patients with advanced clear cell RCC: the ORR was 53% and median PFS was 19.5 months in the 40 mg cabozantinib dose group, and the ORR was 58% and median PFS was 15.1 months for the 60 mg cabozantinib dose group
- Baseline PD-L1+ and high CD8 levels was associated with greater tumor lesion reduction and overall response
- The safety profile of the combination was acceptable at both cabozantinib doses evaluated
- A phase 3 study (CONTACT-03) of cabozantinib with or without atezolizumab in RCC previously treated with immune checkpoint inhibitor therapy is ongoing

Presented by: Sumanta K. Pal, MD, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
Co-Authors: C-K. Tsao,2 C. Suarez,3 W. Kelly,4 L. Pagliaro,5 U.N. Vaishampayan,6 Y. Loriot,7 S. Srinivas,8 B.A. McGregor,9 A. Panneerselvam,10 D. Curran,11 T.K. Choueiri,9 N. Agarwal12
Affiliations: 2 Tisch Cancer Institute, Mount Sinai Hospital, New York, NY, USA, 3 Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Universitat Autònoma de Barcelona, Barcelona, Spain, 4 Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA, 5 Department of Oncology, Mayo Clinic, Rochester, MN, USA, 6 Internal Medicine, Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA, 7 Department of Cancer Medicine, Gustave Roussy Institute, University Paris-Saclay, Villejuif, France, 8 Division of Medical Oncology, Stanford University Medical Center, Palo Alto, CA, USA, 9 Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, USA, 10 Biostatistics, Exelixis, Inc., Alameda, CA, USA, 11 Clinical Development, Exelixis, Inc., Alameda, CA, USA 12 Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
Related Content:
ESMO Virtual Congress 2020: Cabozantinib in Combination with Atezolizumab in Non-Clear Cell Renal Cell Carcinoma: Results from Cohort 10 of the COSMIC-021 Study


