Circulating tumor DNA (ctDNA) analyzed from blood samples provides a means to serially assess tumor genomics without the risks of tissue biopsy. In a prior publication, the authors of this abstract showed that targeted sequencing of circulating tumor DNA can identify genomic alterations in the majority of patients and that ctDNA profiles evolve over time.1
To further explore the information that can be gleaned from ctDNA sequencing, the authors of this presentation identified 847 patients with metastatic RCC who received ctDNA profiling using the Guardant360 platform, a CLIA-certified 74 gene panel. 46 of these patients also had tissue genomic profiling conducted with either exome profiling or targeted sequencing of 324 genes using the Foundation Medicine platform. Results from tissue and ctDNA profiling were compared.
In total, 78.8% of patients had at least 1 genomic alteration detected by ctDNA. The most common genes impacted were TP53, VHL, and EGFR. The authors confirmed their previous finding of evolution in ctDNA profile, as alteration frequencies in various genes changes on serial assessment.
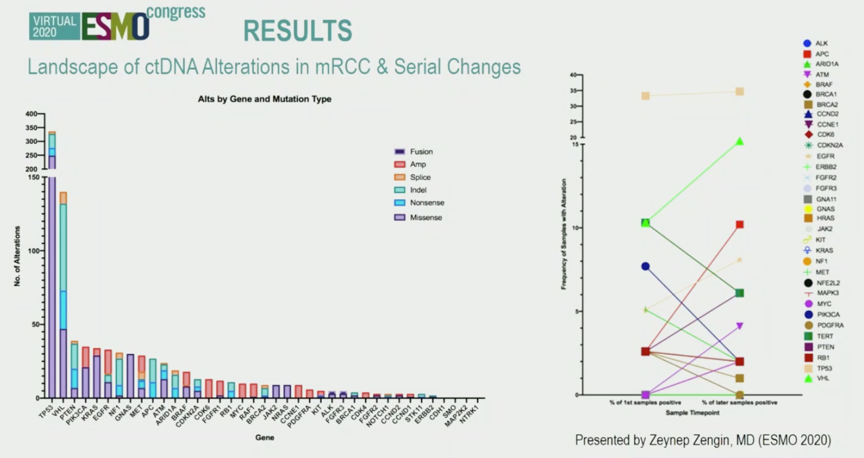
The authors noted that 6% of patients were found to have alterations in DDR genes, including BRCA1, BRCA2, ATM, and CDK12.
The characteristics of the patients with both ctDNA and tissue genomic profiling are shown below.

Notably, the overlap between ctDNA alterations and tissue genomic alterations was low. This is first illustrated by the mutation plot below. Yellow alterations were exclusively found in blood, green alterations were exclusively found in tissue, and blue alterations were found in both.
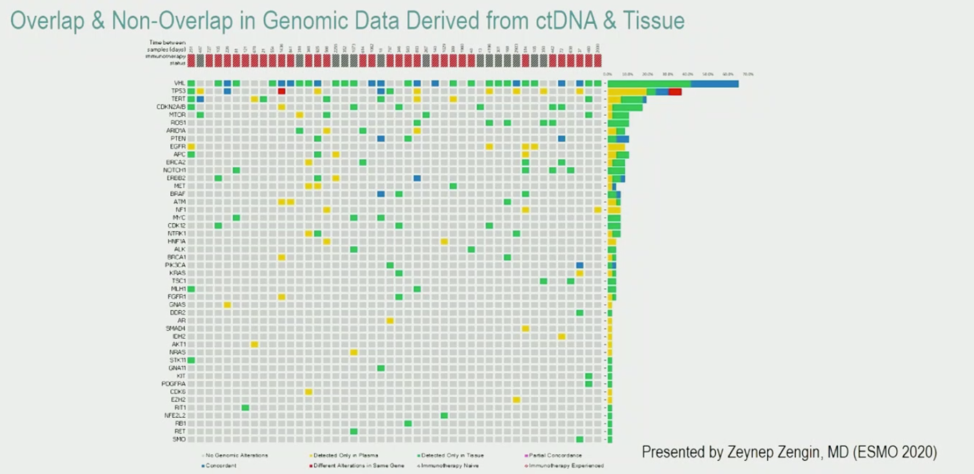
When excluding alterations not covered by one method and excluding patients who did not have detected ctDNA alterations, a total of 21 alterations (22.6% of all alterations) were found in both tissue and blood. The authors hypothesized that this discordance may be due to differences in when the samples were obtained. Indeed, as shown below, the degrees of overlap in alterations found by both ctDNA and tissue profiling increased if the blood and tissue samples were obtained within 6 months of each other. This may suggest that ctDNA captures dynamic changes in the tumor over time.
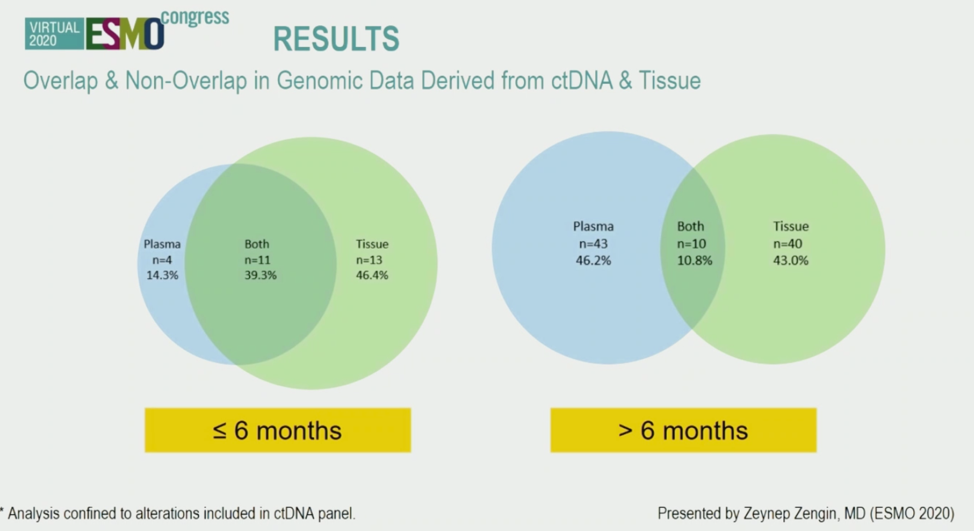
In conclusion, the authors presented what they believe is the largest assessment of ctDNA and mRCC reported to date. This study validated prior findings that ctDNA alterations are detectable in the majority of patients, and these alterations in some cases may be actionable (EGFR mutations for example), though the efficacy of directing therapy in this manner remains to be proven. The concordance between ctDNA and tissue alterations is dependent on time difference between when samples were obtained, and more data is being gathered through collaborations to further understand concordance between these two profiling methods.
Presented by: Zeynep Zengin, MD, Postdoctoral Fellow at the City of Hope Comprehensive Cancer Center, Duarte, CA
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.


