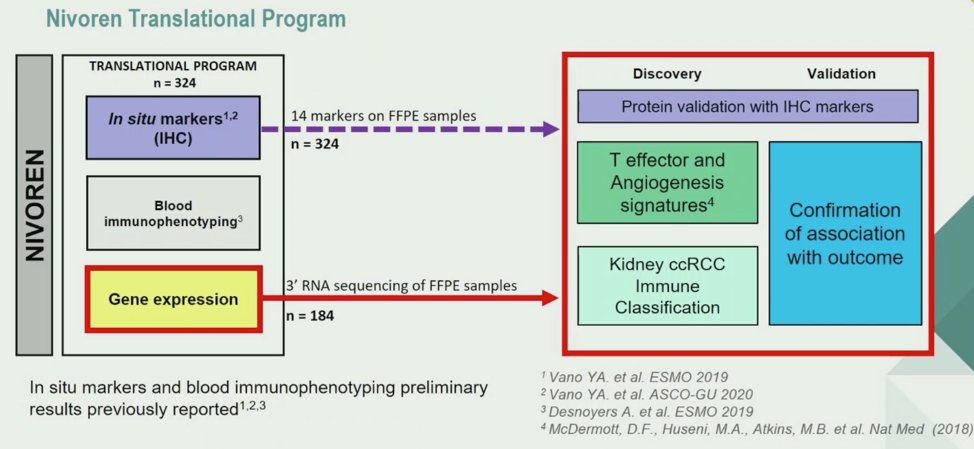
The analysis plan of the NIVOREN translational program is shown below. 14 pre-identified markers were analyzed by immunohistochemistry on formalin-fixed paraffin embedded biopsy tissue. 3’ RNA profiling on FFPE tissue was also conducted on 184 samples to apply previously published gene expression signatures to samples as shown below. Three signatures were utilized: The Teffector and Angiogenesis signatures generated from the IMmotion 150 trial, and a kidney ccRCC immune cell classification (KIC) score to identify the types of cells present in the biopsy specimen. For the latter signature, KIC was first performed by unsupervised clustering, then validated using a separate cohort to predict cell types present.
The characteristics of the 184 patients (divided into the discovery and validation cohorts) are shown below in comparison to the overall NIVOREN cohort. There were differences noted between the percentage of IMDC favorable risk patients as well as the rate of ORR between the discovery and validation cohorts.

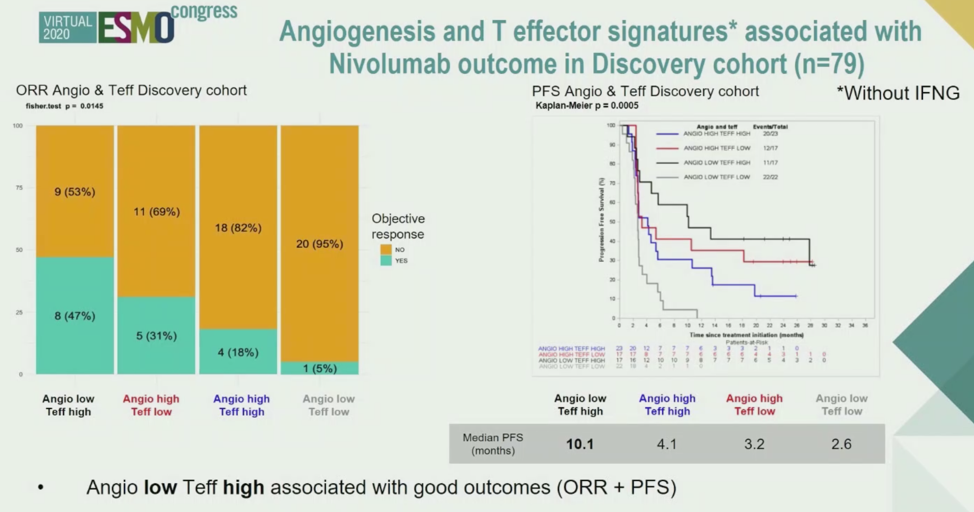
In the discovery cohort, samples with low Angiogenesis signature scores and high Teffector scores were enriched for objective response and had improved progression-free survival relative to other signature combinations, as shown below.
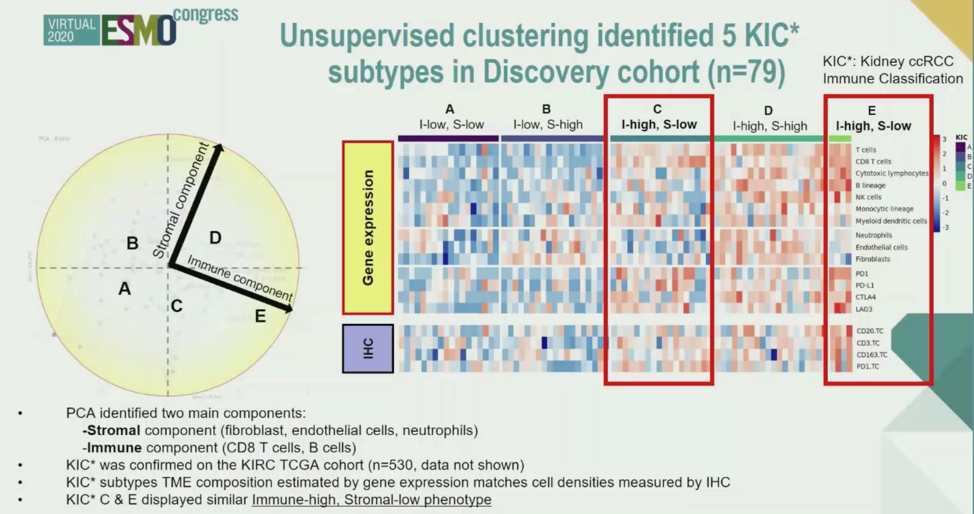
In the discovery cohort, unsupervised clustering using the KIC identified 5 clusters of cell populations within the tumor. These were divided using principal component analysis into high or low stromal cells or high/low immune cells. Immunohistochemistry validated the gene expression classification of cell types present.
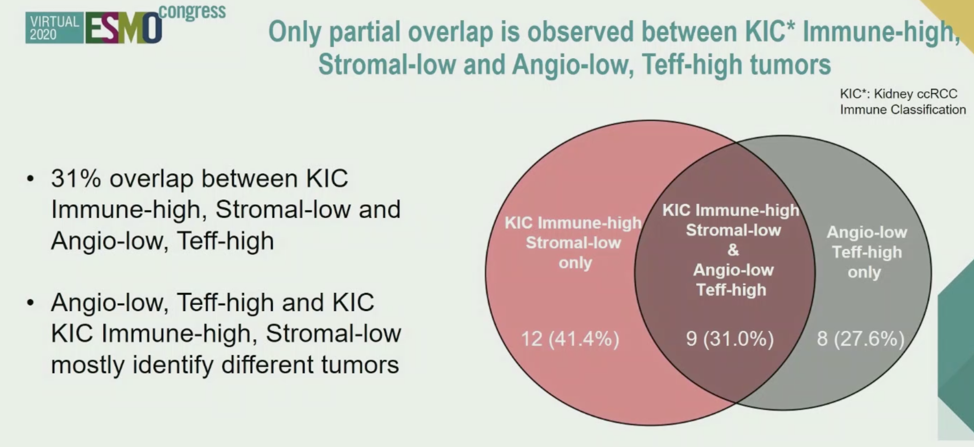
The authors hypothesized that the samples enriched for immune cells (KIC Immune-high) may also have high Teffector and low Angiogenesis gene expression scores. This was not clearly the case, as shown by the Venn Diagram below.
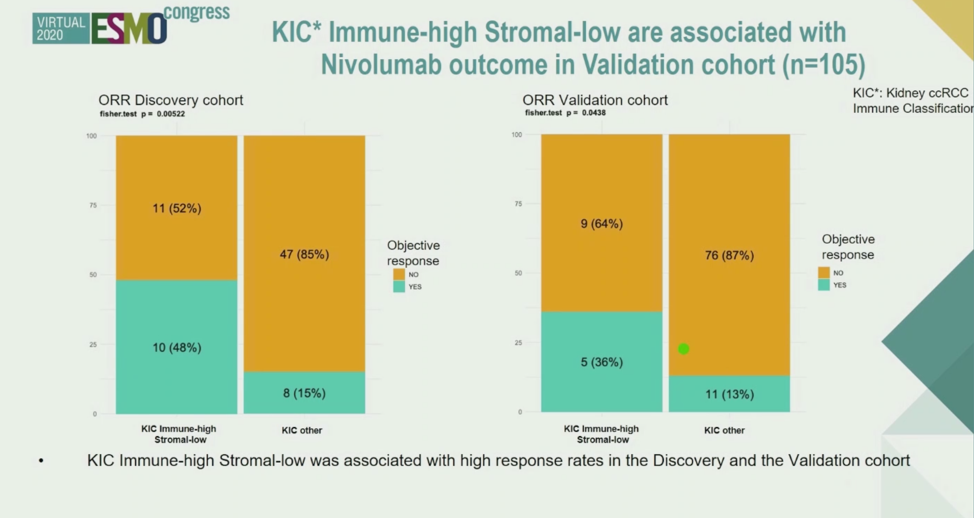
The KIC Immune-High signature was then associated with the outcome. The authors discovered that samples with high scores indicating high levels of immune cells and low levels of stromal cells were most associated with objective response and progression-free survival in the Discovery cohort as well as the Validation cohort.
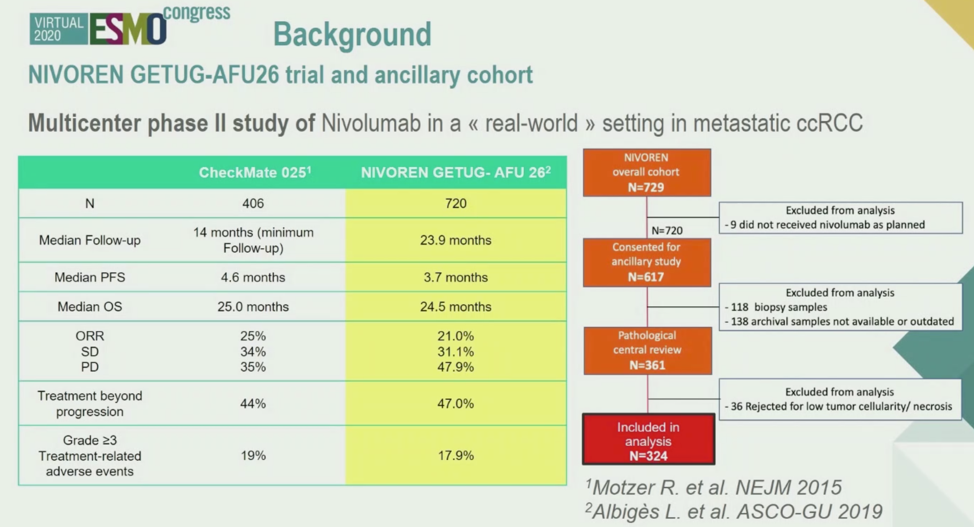
In summary, using samples from the NIVOREN GETUG-AFU26 real-world trial of nivolumab therapy in ccRCC, the authors identified multiple classifications of patient samples based on the application of gene expression signatures. Samples with low scores of the IMmotion150 angiogenesis signature and high scores of the IMmotion150 Teffector signature were most associated with good outcomes. Samples with Immune-high and stromal-low cell populations as assessed by KIC also were associated with relatively superior outcomes. However, the sample overlap between Teffector high/angiogenesis low and KIC Immune-high/stromal-low samples was not absolute, suggesting that multiple biomarkers may be required to predict response to immunotherapy in ccRCC. Most importantly, the immunohistochemistry findings correlated well with the KIC signatures, suggesting that IHC may be useful as a predictive tool.
Presented by: Maxime Meylan, PhD Student at the Cordelier Research Center, the Sorbonne, Paris, France
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
Related Content:
ASCO GU 2020: Comparing Nivolumab versus Everolimus with >5 years of Follow-up in Patients with Advanced Renal Cell Carcinoma: A Final Analysis of the CheckMate 025 Trial


