The ARCHES trial was initially presented at the 2019 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, demonstrating improved radiographic progression-free survival (hazard ratio 0.39, 95% confidence interval 0.30 to 0.50) for patients who received enzalutamide and androgen deprivation therapy (ADT) as compared to ADT alone, though overall survival data were immature. Subsequently, the ENZAMET trial was published demonstrating improved overall survival for men treated with enzalutamide, in addition to androgen deprivation therapy, as compared to ADT alone in men with mHSPC.
In a poster presentation at this year’s European Society of Medical Oncology (ESMO) 2020 Virtual Annual Meeting, Dr. Antonio Alcaraz presented a post-hoc analysis of the ARCHES trial with stratification by baseline prostate-specific antigen (PSA) levels.
To briefly recap, the ARCHES trial randomized men with mHSPC to enzalutamide plus ADT vs placebo plus ADT. Prior to, baseline patients could have ADT treatment and up to six cycles of docetaxel. This presentation represents a post hoc analysis of the effect of baseline PSA on the efficacy of enzalutamide in this disease space.
Among 1,150 enrolled patients, the vast majority (>90%) had received pre-treatment with ADT and 18% had previously received docetaxel. However, median ADT duration prior to randomization was quite short with a median of 1.6 months (range 0.03 to 55.3) among those randomized to enzalutamide and 1.6 months (range 0.03 to 198.8) and those randomized to placebo.
Baseline PSA data were available for 1,146 patients. Of these, 135 patients had PSA ≤0.2 ng/mL, 388 had PSA 0.2-4 ng/mL, and 623 had PSA >4 ng/mL at baseline. The authors found that the relative benefit of enzalutamide to placebo in terms of radiographic progression-free survival was relatively consistent across these three strata of baseline PSA values.
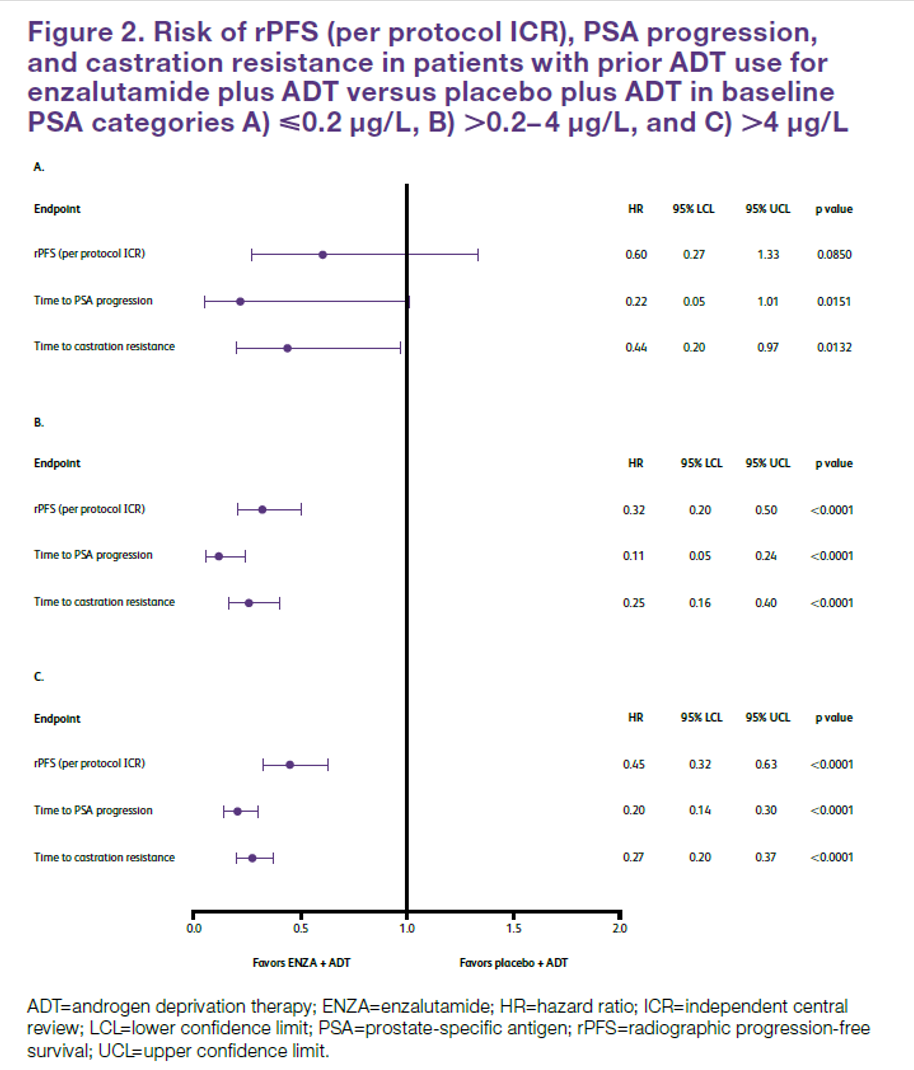
A similar effect was observed examining other endpoints including time to PSA progression and time to castration resistance.
Presented by: Antonio Alcaraz, Head of the Service of Urology and Kidney Transplantation at Hospital Clínic de Barcelona.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center (@WallisCJD) at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020
Related Content:


