In a poster presentation at this year’s European Society of Medical Oncology (ESMO) 2020 Virtual Annual Meeting, Dr. George presented an analysis examining the toxicity of these agents. Important in understanding the risk/benefit trade off of the use of these agents is that, typically, patients with nmCRPC are asymptomatic but at high risk of metastases which may be symptomatic and lead to reduced quality of life.
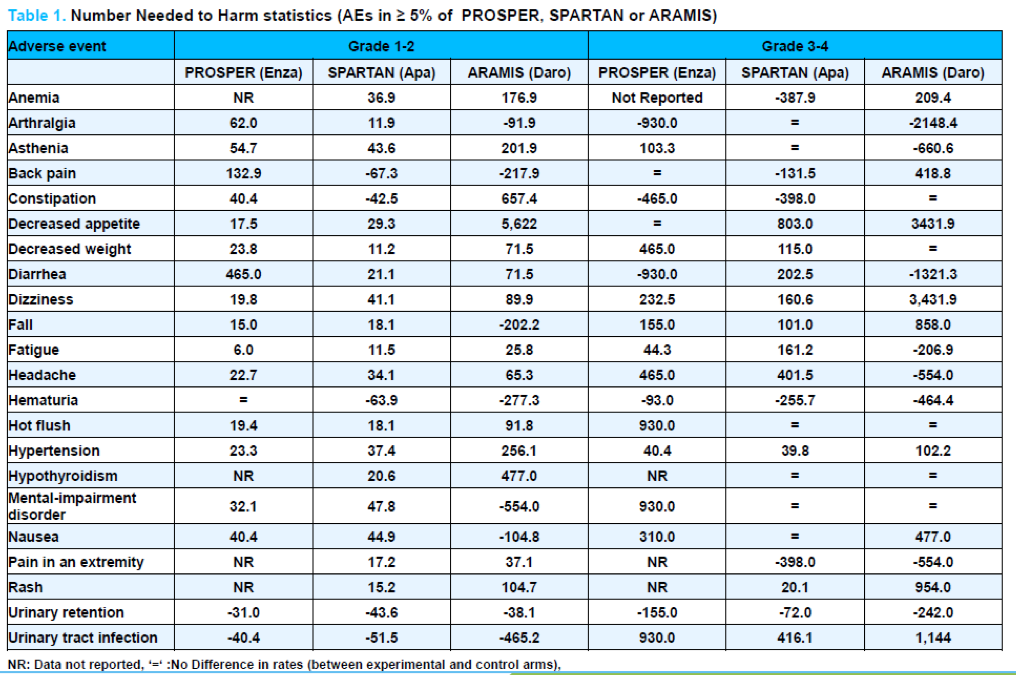
Dr. George and colleagues performed a search of MEDLINE and EMBASE to identify RCTs of enzalutamide, apalutamide, or darolutamide. The authors extracted adverse events that occurred at ≥5% rate in any of the trials. They subsequently calculated the number needed to harm due to adverse events (with stratification according to the grade of toxicity (1-2 vs 3 or greater). They then extrapolated these results to a European population.
As may be expected, the number needed to harm was much higher for serious adverse events (grade 3 and greater) than for grade 1 or 2 events. For some outcomes, number needed to harm was negative, indicating higher risk of this toxicity among patients in the control (rather than experimental) arm of a given trial.
In their extrapolation to the European population, the authors calculated the potential increase in number of adverse events among an estimated 4300 patients with high-risk nmCRPC. As seen in the figure below, the authors estimated that the increase in toxicity associated with adding a novel oral androgen axis inhibitor to ADT for patients with nmCRPC would be minimized with the use of darolutamide.
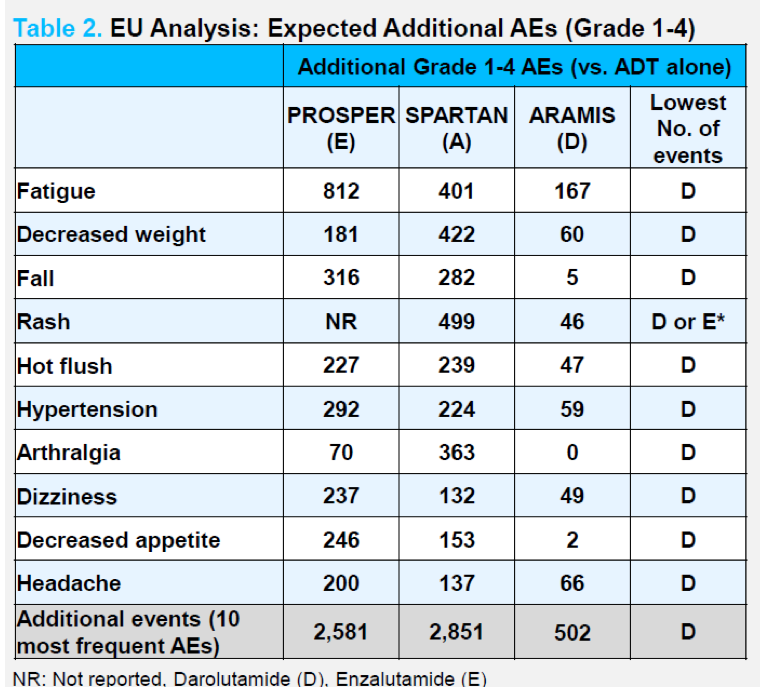
In conclusion, the authors suggest that use of number needed to harm can help to contextualize the risk of adverse events and guide treatment decision making.
Presented by: Daniel J. George, MD, Professor of Medicine and Surgery, Divisions of Medical Oncology and Urology in the Duke University School of Medicine and leads the Duke Prostate and Urologic Cancer Center.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center (@WallisCJD on Twitter) at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020


