In a poster presentation at this year’s European Society of Medical Oncology (ESMO) 2020 Virtual Annual Meeting, Dr. Stéphane Oudard presented an analysis examining the health-related quality of life among men in the SPARTAN trial based on the final data analysis of this cohort.
To reiterate, the SPARTAN trial randomized 1207 patients in a 2:1 fashion to apalutamide 240mg PO daily or placebo. The authors utilized the Functional Assessment of Cancer Therapy–Prostate (FACT-P) and EQ-5D-3L to measure health-related quality of life. These measures were collected are baseline and day 1 of cycle 1 (cycle defined at 28 days), cycles 2-6, every 2 cycles from 7-13, every 4 cycles thereafter during treatment, at the end of treatment, and every 4 months post-progression for up to 1 year. The authors used mixed models for repeated measures to assess the least-squares mean changes from baseline.
With a 52 month follow-up, the authors report that, as expected from improvements in metastasis-free survival, patients randomized to apalutamide were on therapy for much longer (median 32.9 months) than those on placebo (median 11.5 months). At baseline, patients were minimally symptomatic and thus had good health-related quality of life. The questionnaire completion was good (>90%) in both groups.
Utilizing least mean squares changes from baseline with mixed models for repeat measures, the authors demonstrated that patients randomized to apalutamide had significantly preserved health-related quality of life compared to those randomized to placebo (at cycle 21 p=0.014, at cycle 25p=0.0009).
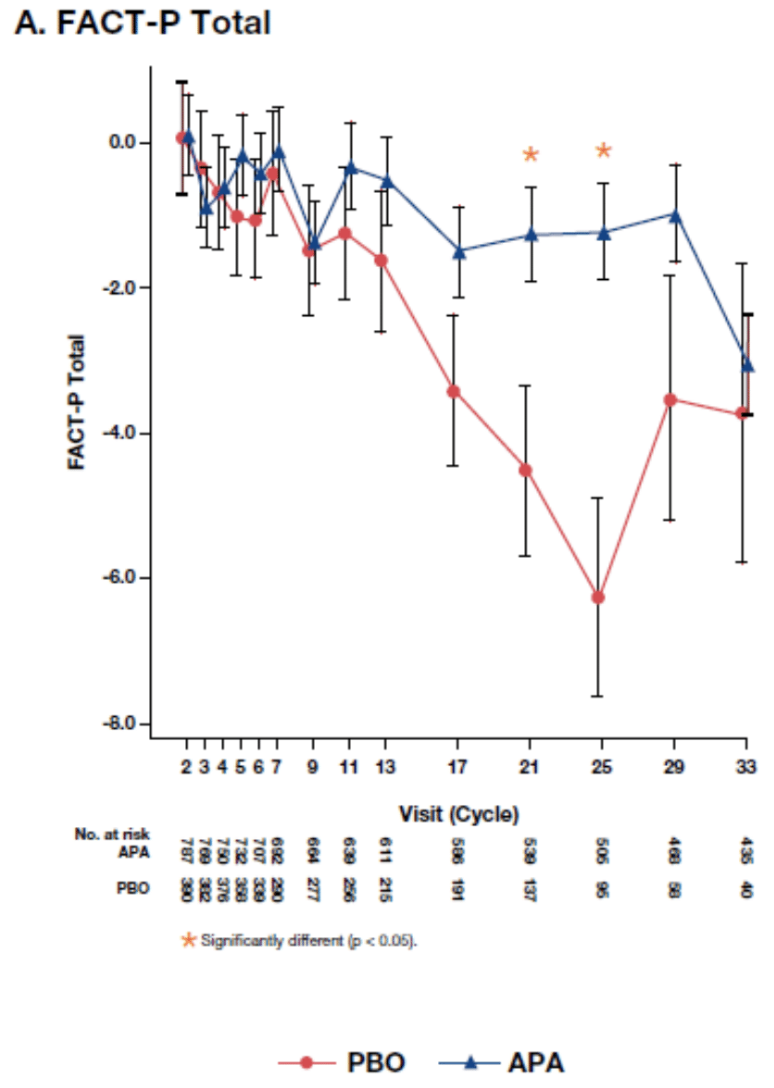
Similar results were observed for the sub-scales of the FACT-P and the EQ-5D-3L with generally preserved health-related quality of life for those on apalutamide and declines for those randomized to placebo.
Presented by: Stéphane Oudard, MD, Ph.D., Professor of Oncology and Chief of the Oncology Clinical and Translational Research Unit at the Georges Pompidou Hospital in Paris, France.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020.


