For KEYNOTE-365, patients with docetaxel-pretreated mCRPC with disease progression within 6 months of screening received pembrolizumab 200 mg IV Q3W plus olaparib 400-mg capsule or 300-mg tablet orally twice daily. Patients could have received one other chemotherapy and ≤2 second-generation androgen receptor-targeted therapies. The study schema for cohort A is as follows:

The primary end points were PSA response rate (decrease ≥50% from baseline), objective response rate (ORR) per RECIST v1.1 by blinded independent central review, and safety. The secondary end points included DCR, composite response rate, DOR, rPFS per PCWG-modified RECIST, overall survival (OS), time to symptomatic skeletal event, radiographic bone progression, and radiographic soft tissue progression. The database cutoff date was June 24, 2019.
For cohort A, 84 of 87 enrolled patients were treated, including 48 of 84 (57.1%) that had measurable disease. The median time from enrollment to data cutoff was 3.6 (range: 0.0-29.2) months for all patients and 26.7 (range: 21.2-29.2) months for 41 patients with ≥27 week follow-up. Confirmed PSA response rate in 82 patients with PSA at baseline was 8.5%:
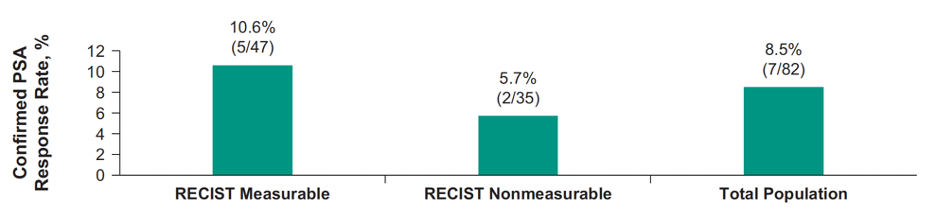
The median time to PSA progression was 3.8 months (95% CI 2.9-4.4). Furthermore, the confirmed ORR in patients with measurable disease and ≥27 week potential follow-up (n = 24) was 8.3% (95% CI 1.0-27.0) (2 partial responses):

Median rPFS was 4.3 months (95% CI, 3.4-7.7) and median OS was 14.4 months (95% CI, 8.1-18.5):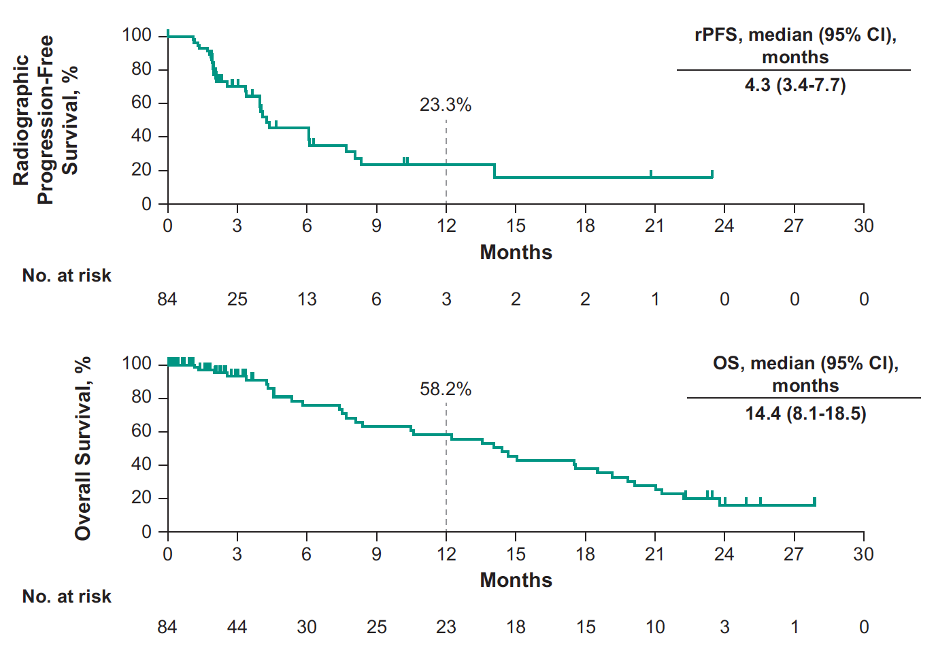
At 12 months, the rPFS rate was 23.3% and OS rate was 58.2% by Kaplan-Meier. The median time to radiographic bone progression was 6.5 (95% CI 4.1-16.1) months, median time to radiographic soft tissue progression was 21.7 (95% CI 5.9-NR) months, and median time to symptomatic skeletal-related event was not reached (95% CI 13.8-NR) months. Grade ≥3 treatment-related adverse events occurred in 29 patients (35%), including w patients that died of treatment-related adverse events (1 myocardial infarction, 1 unknown).
To conclude this update of cohort A of the KEYNOTE-365 trial, Dr. Arija provided the following summary statements:
- With increased enrollment and additional follow-up, pembrolizumab plus olaparib continued to show promising activity in a molecularly unselected mCRPC patient population previously treated with chemotherapy and NHA therapies
- The safety and tolerability profile of pembrolizumab plus olaparib combination therapy was consistent with the individual profiles of each agent
- The promising rPFS and OS data support further evaluation of pembrolizumab plus olaparib in molecularly unselected patients with mCRPC previously treated with docetaxel, with ≤1 other chemotherapy, and with 1 or 2 NHAs
- A randomized phase 3 study of pembrolizumab plus olaparib is open to enrollment for molecularly unselected patients with mCRPC who were pretreated with enzalutamide (for mCRPC) or abiraterone acetate (for metastatic hormone-sensitive prostate cancer or mCRPC) and experienced progression on docetaxel chemotherapy (KEYLYNK-010)
Presented by: Jose A. Arranz Arija, MD, Medical Oncology, General University Hospital Gregorio Marañón, Madrid, Spain,
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020Related Content:
ASCO GU 2020: KEYNOTE-365 Cohort A Updated Results: Pembrolizumab Plus Olaparib in Docetaxel-Pretreated Patients with mCRPC


