For KEYNOTE-365, patients who received abiraterone or enzalutamide for less than or equal to four weeks in the prechemotherapy mCRPC setting and whose disease progressed within six months of screening were eligible. These patients received pembrolizumab 200 mg IV plus docetaxel 75 mg/m2 IV Q3W and prednisone 5 mg orally twice daily. The trial schematic is as follows:
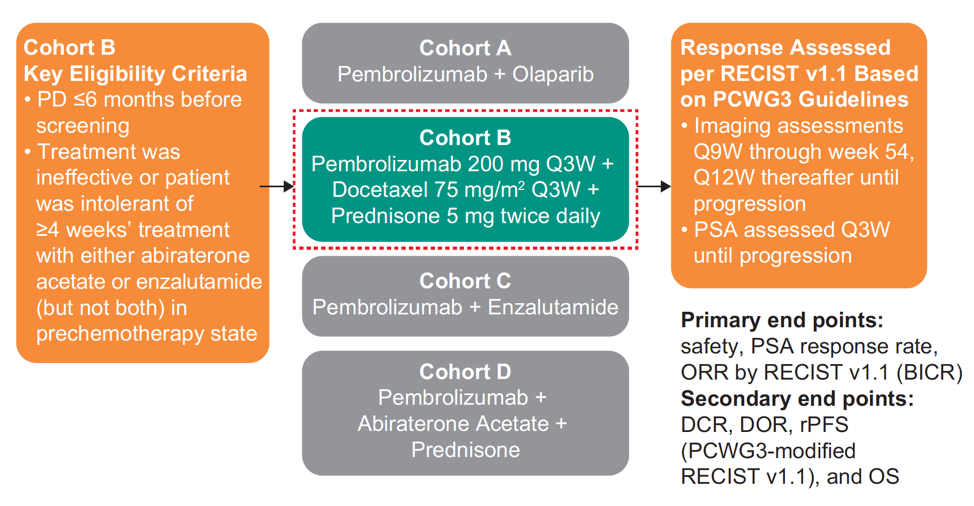
Treatment continued for a maximum of 10 cycles of docetaxel and up to 35 cycles of pembrolizumab (~2 years) or until confirmed disease progression, unacceptable toxicity, or withdrawal of consent. The primary endpoints were PSA response rate (decrease ≥50% from baseline), objective response rate (ORR) per RECIST v1.1 by blinded independent central review, and safety. Secondary endpoints included DCR, DOR, rPFS per PCWG-modified RECIST, OS, time to symptomatic skeletal event, radiographic bone progression, and radiographic soft tissue progression. The database cutoff date was June 24, 2019.
There were 104 of 105 enrolled patients that were treated, approximately 50% of which had measurable disease. The median (range) time from enrollment to data cutoff was 19.9 (1.4-27.8) months for all patients and 21.8 (17.9-27.8) months for patients with ≥27 weeks of follow-up (n = 72). Confirmed PSA response rate was 28.2% in 103 patients with baseline PSA assessment, and 23.5% among patients with RECIST measurable disease. Additionally, for patients with measurable disease and ≥27 weeks of follow-up (n = 39), confirmed ORR was 18% (7/39, all partial response):

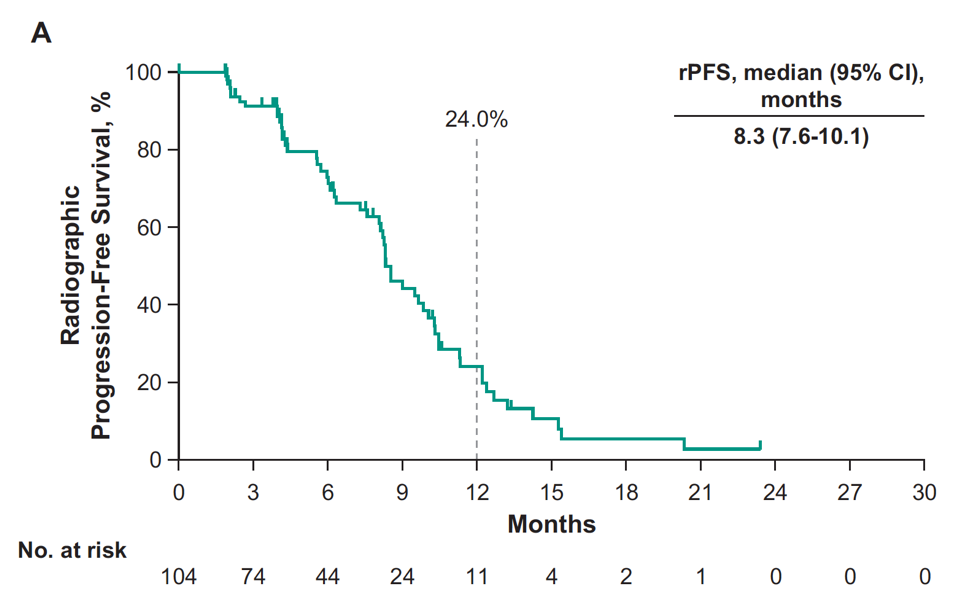
For all patients, the median rPFS was 8.3 months (95% CI, 7.6-10.1):
and the median OS was 20.4 months (95% CI, 16.9-NR):
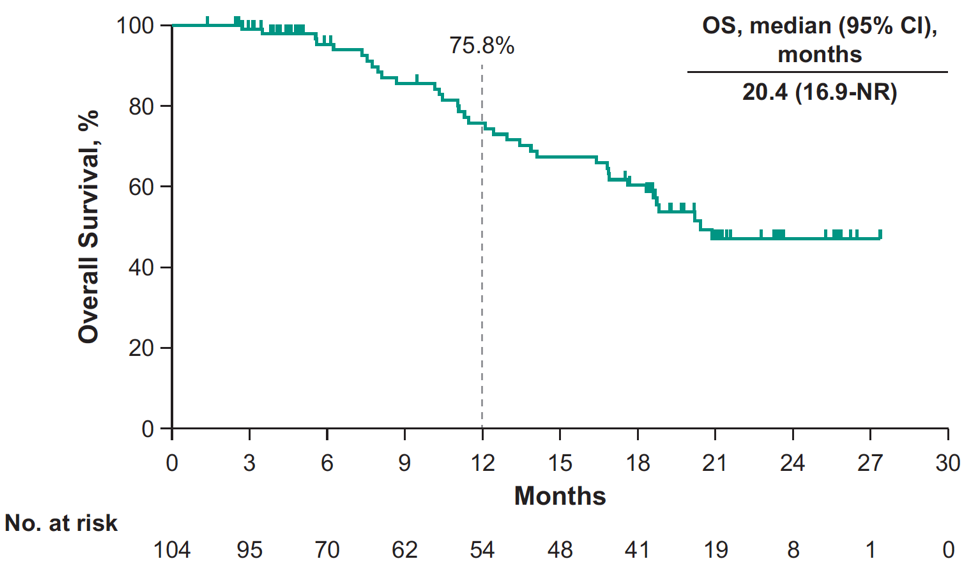
At 12 months, the rPFS rate was 24.0% and OS rate was 75.8% by Kaplan-Meier. Median time to radiographic bone progression was 10.3 (95% CI 9.0-12.2) months, median time to radiographic soft tissue progression was 12.4 (95% CI 9.7-NR) months, and median time to symptomatic skeletal-related event was 25.9 (95% CI 18.3-NR) months. Grade ≥3 treatment-related adverse events occurred in 40% of patients, including two patients that died of pneumonitis.
Dr. Romano concluded this update of cohort B of the KEYNOTE-365 with the following summary points:
- With increased enrollment and additional follow-up, pembrolizumab plus docetaxel plus prednisone continued to show promising activity in patients previously treated with abiraterone acetate or enzalutamide in the prechemotherapy state of mCRPC
- The safety of the combination was consistent with the known profiles of the individual agents
- The promising data from this study support further evaluation of docetaxel plus prednisone with or without pembrolizumab in patients with mCRPC previously treated with abiraterone acetate or enzalutamide
- A randomized phase 3 study of docetaxel plus prednisone with or without pembrolizumab is open to the enrollment of patients with mCRPC who are chemotherapy naïve and were previously treated with one (but not more than one) next-generation hormonal agent for metastatic hormone-sensitive prostate cancer or CRPC (KEYNOTE-921)
Presented by: Emanuela Romano, Medical Director, Medical Oncology, Center for Cancer Immunotherapy, Institut Curie, Paris, France
Co-Authors: S.S. Sridhar,2 M.P. Kolinsky,3 G. Gravis,4 L. Mourey,5 J.M. Piulats,6 W.R. Berry,7 H. Gurney,8 M. Retz,9 L.J. Appleman,10 M. Boegemann,11 J.S. de Bono,12 A.M. Joshua,13 U. Emmenegger,14 H.J. Conter,15 B. Laguerre,16 H. Wu,17 P. Qiu,17 C. Schloss,17 E.Y. Yu18
Author Affiliations:
2 Cancer Clinical Research Unit, Princess Margaret Cancer Centre, Toronto, ON, Canada, 3 Medical Oncology, Cross Cancer Institute, Edmonton, AB, Canada, 4 Translational Medicine, Institut Paoli-Calmettes, Paris, France, 5 Urology, Insitut Universitaire du Cancer–Oncopole, Toulouse, France, 6 Medical Oncology, Catalan Institute of Oncology, Barcelona, Spain, 7 Medical Oncology, Duke Cancer Center Cary, Cary, NC, USA, 8 Medical Oncology, Macquarie University Hospital, Sydney, Australia, 9 Department of Urology, Rechts der Isar University Hospital, Technical University of Munich, Munich, Germany, 10 Division of Hematology & Oncology, UPMC, Pittsburgh, PA, USA, 11 Department of Urology, Muenster University Hospital, Muenster, Germany, 12 Medical Oncology, The Royal Marsden NHS Foundation Trust, London, UK, 13 Medical Oncology, Kinghorn Cancer Centre - St Vincent’s Hospital, Sydney, Australia, 14 Medical Oncology, Sunnybrook Research Institute, Toronto, ON, Canada, 15 Medical Oncology/Hematology, University of Western Ontario, London, ON, Canada, 16 Medical Oncology, Centre Eugène Marquis, Rennes, France, 17 Medical Oncology, Merck & Co., Inc., Kenilworth, NJ, USA 18 Department of Medicine, Division of Oncology, University of Washington, Seattle, WA, USA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020
References:
Related Content:
ASCO GU 2020: KEYNOTE-365 Cohort A Updated Results: Pembrolizumab Plus Olaparib in Docetaxel-Pretreated Patients with mCRPC


