(UroToday.com) At this year’s European Society of Medical Oncology (ESMO) 2020 Virtual Congress, following two abstracts assessing the role of metformin and local prostate-directed therapy in patients with newly diagnosed, hormone-sensitive metastatic prostate cancer, Dr. Noel Clarke provided an invited discussion of these data.
He first discussed the MANSMED trial assessing metformin in patients with advanced prostate cancer in a randomized design. He highlighted that these included patients with high-risk hormone-sensitive localized or locally advanced disease and metastatic hormone-sensitive disease.
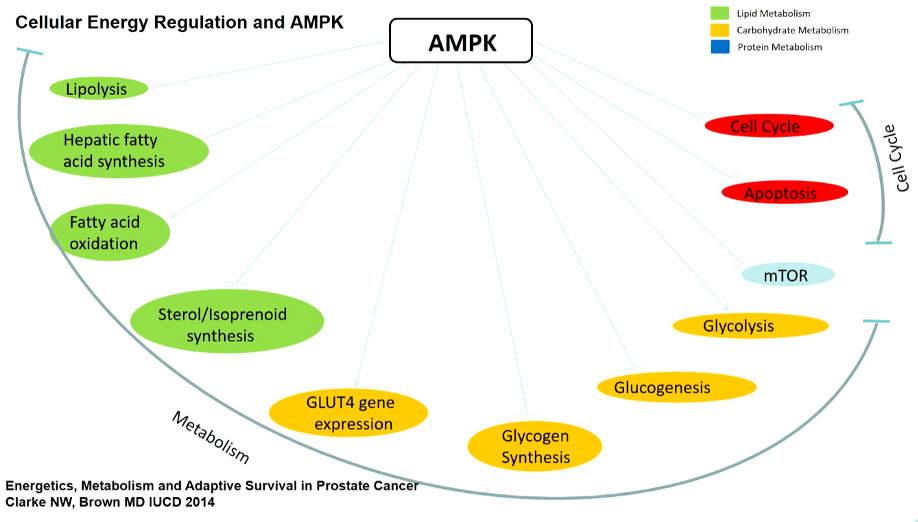
He began by highlighting data assessing metformin and anti-cancer effects. Among patients with diabetes, systematic reviews have demonstrated a benefit in terms of cancer-specific mortality rates among those receiving metformin. Mechanistically, there are putative benefits of metformin due to the slowing down of energy utilization and metabolic pathways. By enhancing the effects of AMPK, metformin can impede metastatic transition through the epithelial-mesenchymal transition.
Coming back to the MANSMED trial, Dr. Clarke highlighted significant separation in the Kaplan Meier curves for the primary endpoint of castration-resistant free survival. His concern, however, is that these findings are derived among a very small sample of patients (62 patients in each of the intervention and control arms). Thus, he considered these randomized data to be still hypothesis-generating rather than conclusive. However, the STAMPEDE trial has already recruited 2200 patients to Arm K to the standard of care plus metformin. These data are likely to prove to be the more conclusive data on the subject.
Moving to the next abstract (618), Dr. Clarke discussed a phase II trial of local therapy in men with oligometastatic prostate cancer. As most readers will know, the STAMPEDE-RT and HORRAD trials demonstrated improved overall survival for patients receiving radiotherapy to the prostate among those with low volume disease, despite no benefit in the overall study population.
Among patients with newly diagnosed oligometastatic prostate cancer, these authors assessed treatment with either radical prostatectomy or radiotherapy in addition to standard ADT. The patients represent an expected disease population. Dr. Clarke highlighted that median PSA was quite high (85 ng/mL), as was grade with 86% having Gleason score 8 or greater, and more than 80% having T3a or greater stage. Over a median follow-up of 28 months, the authors demonstrated a “striking” improvement in radiographic progression-free survival. Again, he considered this data to be strong phase II data but still hypothesis-generating. Again, Dr. Clarke emphasized that, in his view, larger studies are needed. Again, within STAMPEDE, Arm M will assess radiotherapy to the prostate as well as to oligometastatic sites with 900 patients per arm.
Presented by: Noel William Clarke, MD, Consultant Urologist at Salford Royal Hospital and The Christie, Manchester, United Kingdom
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
Related Content:
Treating the Primary in High vs Low Volume Hormone Sensitive Disease - Interview Noel Clarke
APCCC 2019: Local Treatment of The Primary (Surgery) in the Metastatic Situation


