- The host: comorbidities, biologic age, patient preference
- The tumor: pathology, morphology, and extent of disease
- The therapeutic agents: historically one size fits all regarding efficacy, but with differences in safety
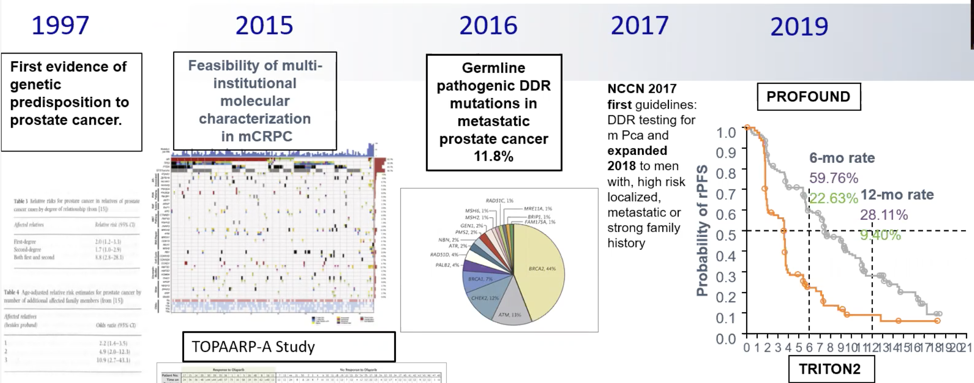
Dr. Efstathiou notes that there will be effort required in prostate cancer treatment to shift the germline testing “culture”:
- We need system-wide integration of germline testing versus just routine testing (a hit or miss approach)
- We need regimented germline mutation carrier follow-up
- We need systemic molecular characterization of tumors and use this acquired knowledge. Currently, the methodologies remain suboptimal, inconsistent, and expensive.
- We need to dissect how to use the therapeutics available to us: How should prostate cancer patients with DNA damage repair aberrations (somatic or germline) be treated? Should patients still be exposed to other available agents such as chemotherapy and enhanced androgen signaling inhibition?
Dr. Efstathiou then discussed the clinical impact of somatic alterations in prostate cancer patients with and without previously known germline BRCA1/2 mutations (the PROREPAIR-A study) presented by Dr. Mejorada and colleagues. She notes that patients with germline DNA damage repair alterations and more specifically BRCA2 mutations have a poor prognosis for prostate cancer outcomes. In this multicenter case-control study, germline BRCA2 carriers were matched 1:2 to known non-carriers by Gleason score and stage at diagnosis (M0 vs M1). Germline BRCA2 carriers were younger at diagnosis (p=0.02) and had more often T3/4 (p<0.001) disease than non-carriers, but no other significant differences were found. In terms of molecular characteristics, germline BRCA2 carriers presented with more somatic alterations than non-carriers (p<0.001), including BRCA2 loss, RB1 loss, and MYC amplification. BRCA2 was also more frequently co-deleted with RB1 (Pearson correlation 0.96; p=0.001). Germline BRCA2 mutations were independently associated with CSS (HR 3.70; p=0.008). CSS was also shorter in germline BRCA2 carriers who present with somatic BRCA2-RB1 co-deletion or MYC amplification compared with germline BRCA2 without such alterations. A multivariable model confirmed the independent prognostic value of somatic BRCA2-RB1 co-deletion (HR 4.13; p=0.004) and MYC amplification (HR 2.27; p=0.033) for CSS. According to Dr. Efstathiou, this confirms the independent prognostic value of germline BRCA2 mutations for cause-specific survival in prostate cancer patients. She also notes that the BRCA2-RB1 co-deletion appears a commonly found pair of genomic events, and hence is not likely to change the plan of care. Furthermore, MYC amplification coupling to BRCA2 deletion is variable, and it remains to be seen how this impacts the treatment planning and whether it bears any weight in our decision making.
Finally, Dr. Efstathiou discussed the abstract presented by Aldea et al which looked at cabazitaxel activity in men with mCRPC with and without DNA damage repair defects. Based on the data we have, Dr. Efstathiou wonders if we can extrapolate outcomes from germline BRCA to somatic BRCA. The study by Aldea et al looked at 190 patients, 95 of which were DNA damage repair positive and 95 that were DNA damage repair negative. DNA damage repair positive patients were younger than DNA damage repair negative (66 vs 69 years, p=0.026). The Gleason score was ≥8 in 66% and 55%, metastases were found at diagnosis in 51% and 41%, respectively. At the start of cabazitaxel, patients had received a median of two prior life-prolonging agents, visceral metastasis in 24% and 26%, ECOG ≤1 in 78% and 80%, and a median PSA of 91 and 77 ng/ml, respectively. Among DNA damage repair positive patients, 40 (42%) had BRCA defects and 43 (45%) received a PARP inhibitor. A 50% PSA decline was achieved with cabazitaxel in 29 (32%) and 33 (36%) in DNA damage repair positive and DNA damage repair negative patients (p=0.64). Median rPFS was 5.33 months (95% CI 4.34-7.04) for DNA damage repair positive patients and 5.75 months (95% CI 4.67-7.27) (p=0.55) for DNA damage repair negative patients, Median OS was 15.4 months (95% CI 12.16-26.6) for DNA damage repair positive patients and 11.5 months (95% CI 9.76-14.4) (p=0.036) for DNA damage repair negative patients. Dr. Efstathiou notes that prospective validation with rigorous and regimented molecular profiling is warranted. Currently, PARP inhibition is the standard of care for mCRPC DNA damage repair positive patients post enhanced androgen signaling inhibition regardless of chemotherapy exposure, however, based on this retrospective data, cabazitaxel is a valid option to consider if PARP inhibitors are not available (or the patient is a post-PARP inhibitor failure).
Presented by: Eleni Efstathiou, MD, Ph.D., Associate Professor, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
References:
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020 Aug 14 [Epub ahead of print].
ESMO Virtual Congress 2020: Imaging Based Prostate Cancer Screening Among BRCA Mutation Carriers – Results from the First Round of Screening
ESMO Virtual Congress 2020: Clinical Impact of Somatic Alterations in Prostate Cancer Patients with and Without Previously Known Germline BRCA1/2 Mutations: Results from PROREPAIR-A Study
ESMO Virtual Congress 2020: Cabazitaxel Activity in Men with Metastatic Castration Resistant Prostate Cancer with and without DNA Damage Repair Defects


