A total of 629 patients with metastatic prostate cancer were randomized between 2005 and 2013 to Arms A (androgen deprivation therapy (ADT)) or Arm C (ADT and docetaxel) with available scans analyzed. Additionally, a comprehensive lymph node analysis was done using the UK Royal College of Radiologists diagnostic criteria with a central review of CT/MRI scans performed jointly by a radiologist and researcher. All lymph nodes (regional and non-regional) were assessed for size and number. The studied endpoints included overall survival (OS) and failure-free survival (FFS). Using multivariable Cox regression analysis, the authors adjusted for:
- age (<70 or ≥70)
- WHO performance status (0 or 1-2)
- Gleason score (≤7, 8-10 or unknown)
- regional nodal status (N0, N1, or NX)
- bone metastasis counts (<5 or ≥5) concomitant metastatic site (only metastatic lymph nodes, bone (+/-metastatic lymph nodes)
- any visceral/other (+/-Bone))
- NSAID or aspirin use (both/either or no)
Table 1 – Baseline characteristics:
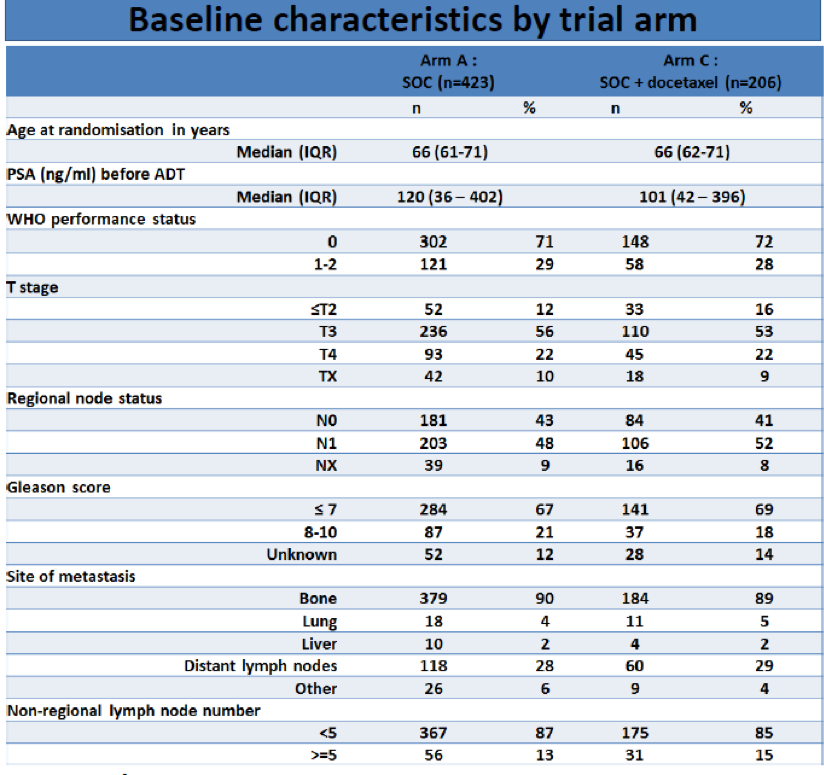
Figure 1 – Lymph node distribution:
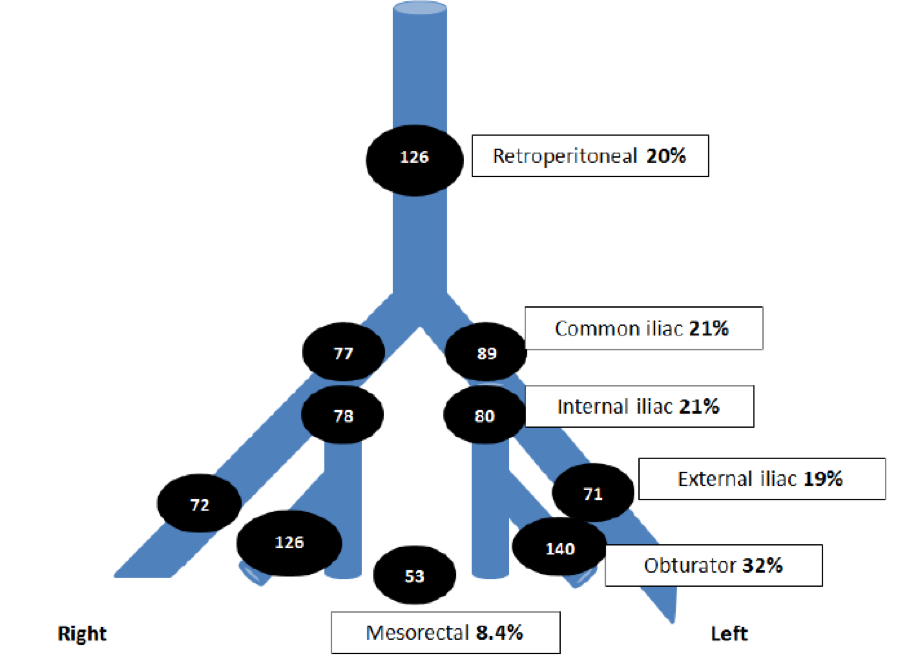
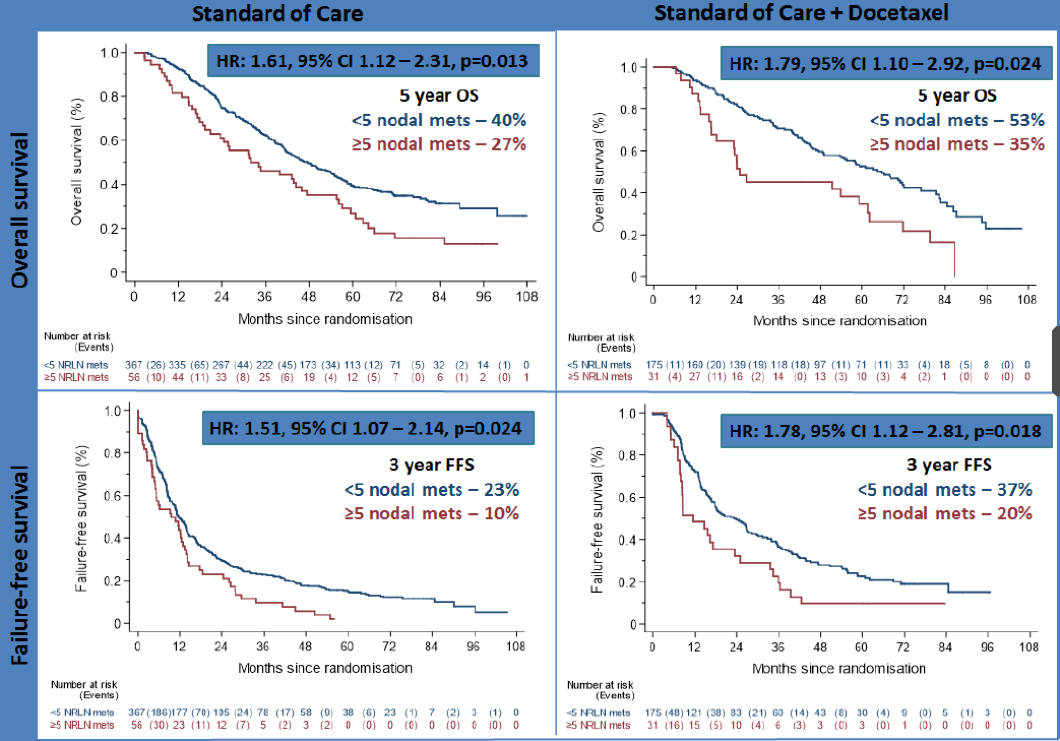
Patients with five or more metastatic nodes had worse overall survival (OS) compared to patients with less than five metastatic nodes in both the ADT group (HR=1.61, 95%CI 1.12–2.31, p=0.013; 5yr KM estimated OS of 27% for ≥five metastatic nodes vs. 40% for <5 metastatic nodes) and the ADT + docetaxel group (HR=1.79, 95%CI 1.10–2.92, p=0.024; 5yr OS of 35% vs. 53%).
Moreover, failure-free survival (FFS) was worse for patients with 5 or more metastatic nodes in the ADT group (HR=1.51, 95%CI 1.07–2.14, p=0.024; 3yr FFS of 10% vs 23%) and the ADT + docetaxel group (HR=1.78, 95%CI 1.12–2.81, p=0.018; 3-yr FFS of 20% vs 37%). The Kaplan -Meyer curves are shown in figure 2.
Figure 2 – Overall survival and failure-free survival:
In conclusion, this study demonstrated that in patients with metastatic hormone-sensitive prostate cancer treated with ADT or ADT and docetaxel, increased metastatic lymph node burden at baseline (≥5 metastatic nodes) was associated with worse overall and failure-free survival outcomes. This should potentially be considered as an independent poor prognostic factor. Due to this presented data, the authors believe that nodal metastases should be included in prospective risk/volume and oligometastatic definitions, to aid risk stratification, future trial design, and treatment for this unique patient cohort.
Presented by: Dr. Aine M. Haran, The Christie and Salford Royal Hospitals, Manchester, UK
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020.
References:


