
At the time of the primary radiographic progression free survival analysis, there was a trend for improvement in overall survival with olaparib compared to the control treatment in Cohort A (patients harboring alterations in BRCA1, BRCA2 or ATM: HR 0.64, 95% CI 0.43-0.97, p=0.02), despite 61% of patients in the control arm switching to receive subsequent olaparib treatment:
In oncology trials, patients switching from the comparator to the experimental treatment may lead to underestimation of the ‘true’ overall survival benefit for the experimental treatment. By adjusting for switching, further insight can be gained into overall survival benefit:
At the virtual European Society of Medical Oncology – 2020 Virtual Congress (ESMO) annual meeting, Dr. Neeraj Agarwal and colleagues presented results of their study assessing the impact of switching on the interim overall survival results for Cohort A in PROfound using five statistical approaches, including simple and more complex methods.
Following guidance from health technology assessment agencies and the European Medicines Agency, the switching-adjustment methods comprised two simple methods of 1) excluding patients in the physician’s choice of next generation hormonal agent arm who received subsequent olaparib, 2) censoring patients in the physician’s choice of next generation hormonal agent arm on receiving subsequent olaparib, and three more complex methods of 3) rank preserving structural failure time model (RPSFTM), 4) inverse probability of censoring weights (IPCW), and 5) two-stage estimation (TSE).
As mentioned above, at the interim analysis, the overall survival hazard ratio in Cohort A was 0.64 (95% CI 0.42–0.97), with 61% of patients randomized to physician’s choice of next generation hormonal agent having switched to olaparib. With simple methods, the switching-adjusted HRs (95% CI) ranged from 0.36 (0.20–0.64) to 0.63 (0.35–1.13). The more complex methods of RPSFTM and IPCW yielded HRs of 0.41 (0.22–0.77) to 0.52 (0.28–0.95); the TSE method could not be reliably performed as a suitable secondary baseline was not identified. A summary of these findings is as follows: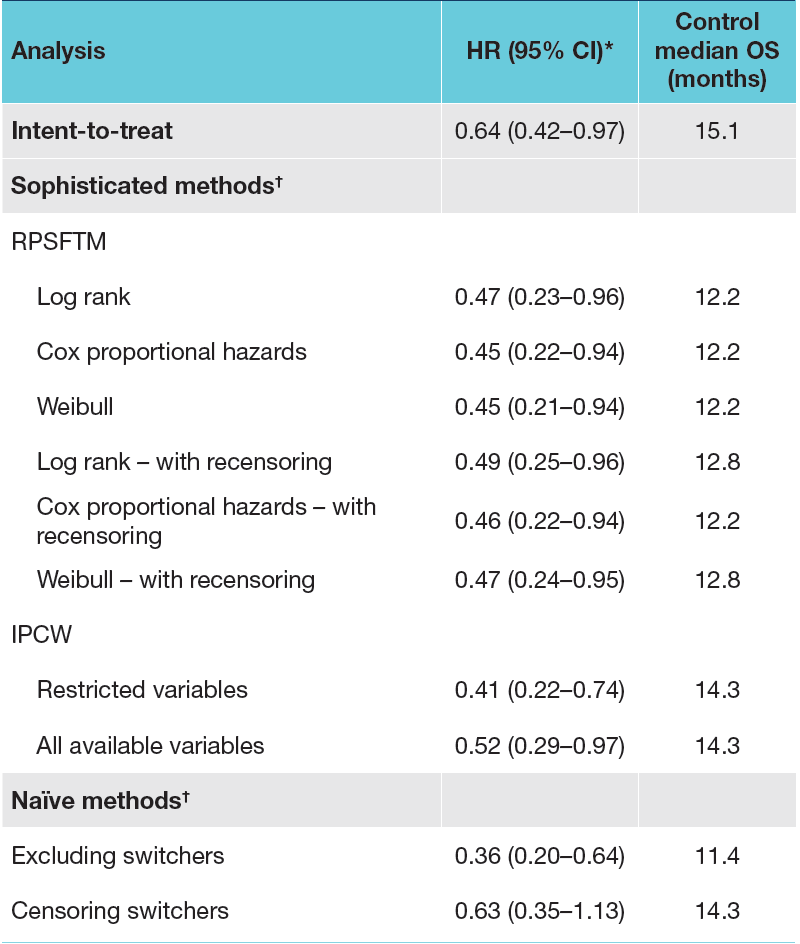
The RPSFTM method was determined to be the most appropriate approach for the PROfound study, considering the assumptions of the method. The randomization assumption that the two groups are comparable, and that if they had both received control treatment their survival outcomes would be the same on average, were shown to hold in the analysis. The common treatment effect assumption was tested through a sensitivity analysis whereby a proportion of the olaparib treatment effect was applied to those switching to olaparib from the control arms, which had minimal impact on the results, suggesting that the analysis is robust to changes in treatment effect over time. As follows are Kaplan-Meier estimates of the interim overall survival in Cohort A with adjustment for treatment switching from control therapy to olaparib using the RPFSTM approach, showing reduced HRs for death compared with the unadjusted analysis (HR 0.64):
Dr. Agarwal conclude this presentation of the PROfound study with the following summary statements:
- Statistical modelling with RPSFTM was identified as the most appropriate approach to account for treatment switching in the PROfound study
- Adjustment for treatment switching from the control therapy to olaparib in Cohort A yielded a reduction in the hazard ratio estimate for overall survival from 0.64 (unadjusted) to 0.45-0.49 using the preferred RPSFTM approach, suggesting that the overall survival benefit for olaparib was greater than was observed at the interim analysis
- Although the IPCW was not considered the best approach to adjust for treatment switching using the PROfound study data, the findings provided clinically credible results that are consistent with those achieved with RPSFTM, despite using different assumptions
- These data are limited by the relatively small sample size of the control arm of Cohort A and the small proportion of patients who did not switch to olaparib treatment, as well as the assumptions required for each method
- This analysis shows that the unadjusted intention to treat estimate of the treatment effect in the PROfound study may be underestimated, and that when switching is adjusted for, the benefit from treatment with olaparib for these patients with mCRPC could be even greater for both Cohort A and the overall population
- This modeling is being assessed for the PROfound study final overall survival data (the final overall survival data is being presented at the Presidential Symposium at ESMO 2020)
Presented by: Neeraj Agarwal, Oncology/Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020
Related Content:
Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer


