The addition of abiraterone acetate plus prednisone (AAP) to androgen deprivation therapy (ADT) in the phase 3 LATITUDE study (Figure 1) showed significant survival benefits in patients with newly diagnosed high-risk mHSPC1.
In this presented poster, a subgroup analysis of the phase 3 LATITUDE study was conducted to assess the impact of AAP on OS and radiographic progression-free survival (rPFS), specifically in mHSPC patients with visceral metastases.
Figure 1 – LATITUDE study design:

In this sub-group analysis presented, patients with visceral metastases in the liver or lungs with or without other soft tissue and bone metastases (based on CT/MRI) at baseline were included. The coprimary efficacy endpoints, OS, and rPFS were assessed using the Kaplan-Meier method. A non-stratified Cox regression model was used to estimate hazard ratios (HR) and 95% CI.
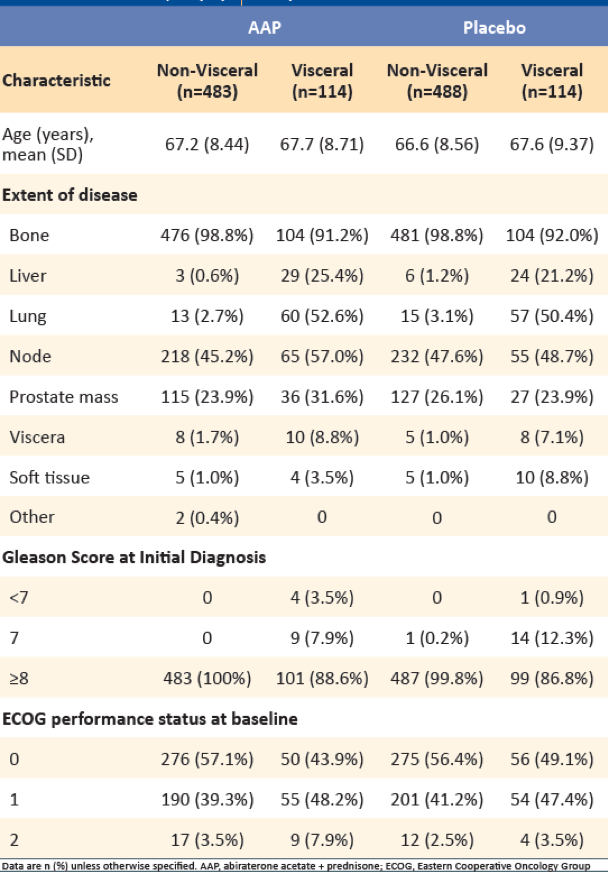
Among 1199 patients with mHSPC, who received either AAP + ADT or placebo + ADT, 228 (19%) had visceral metastases (114 in each group). Of the 228 patients with visceral metastases, liver metastases were reported in 53/228 (AAP: 29, Placebo: 24) patients, and lung metastases were reported in 117/228 (AAP: 60, Placebo: 57) patients. A total of 72 patients from the placebo group (non-visceral: 61 and visceral: 11) had crossed over to the AAP treatment group. Table 1 demonstrates the baseline and demographic characteristics stratified by the presence of visceral metastases.
Table 1 – Baseline demographic characteristics:
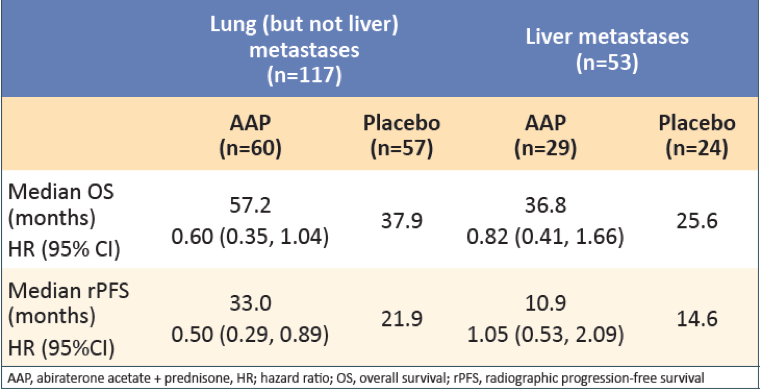
In patients with visceral metastases, AAP showed greater improvement in OS and rPFS compared with placebo (Table 2). Treatment with AAP vs. placebo was associated with greater improvement in OS in patients with lung metastases than in patients with liver metastases. Treatment with AAP vs. placebo was also associated with improved rPFS in patients with lung metastases, but no improvement was shown in rPFS for patients with liver metastases (Table 3).
Table 2 – Overall survival results in patients with visceral disease:
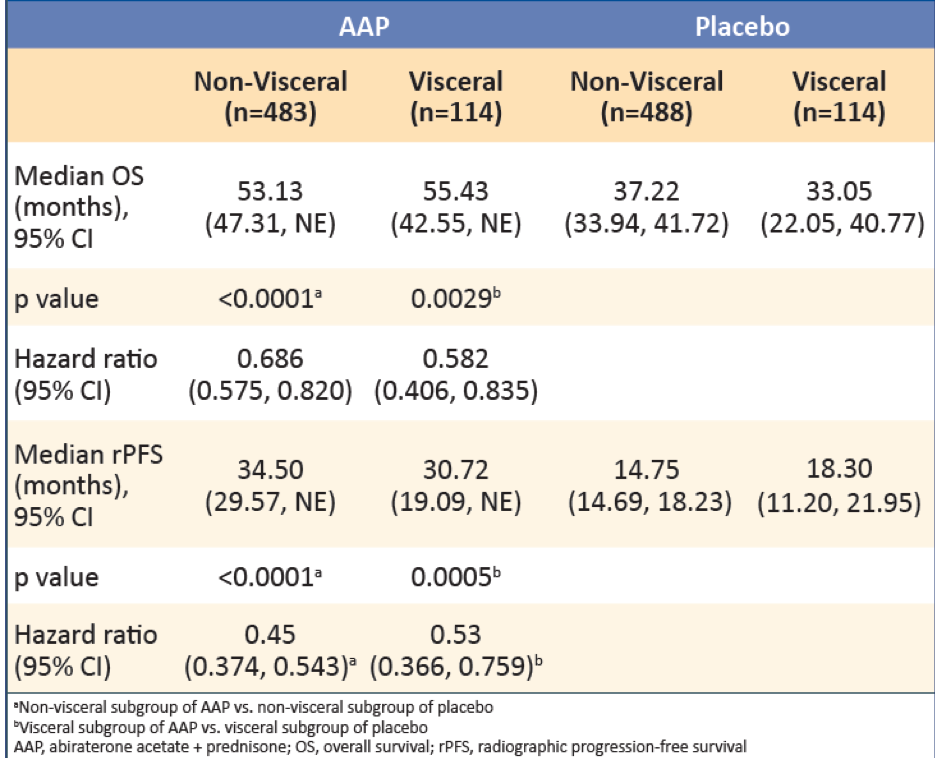
Table 3 – Overall survival and rPFS in patients with visceral disease:
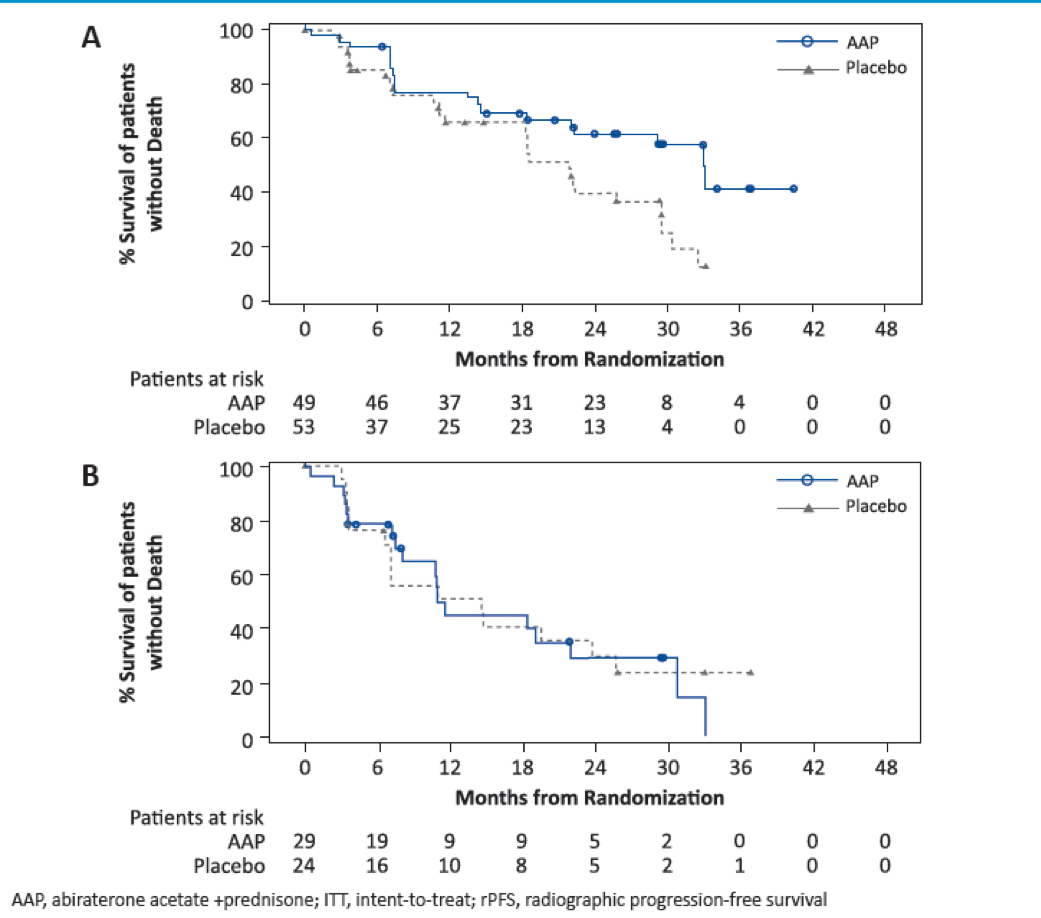
Figures 2 and 3 show the Kaplan Meier plots of OS and rPFS in patients with lung and liver metastases, respectively.
Figure 2 – Kaplan Meier plot of overall survival in lung and liver metastases:
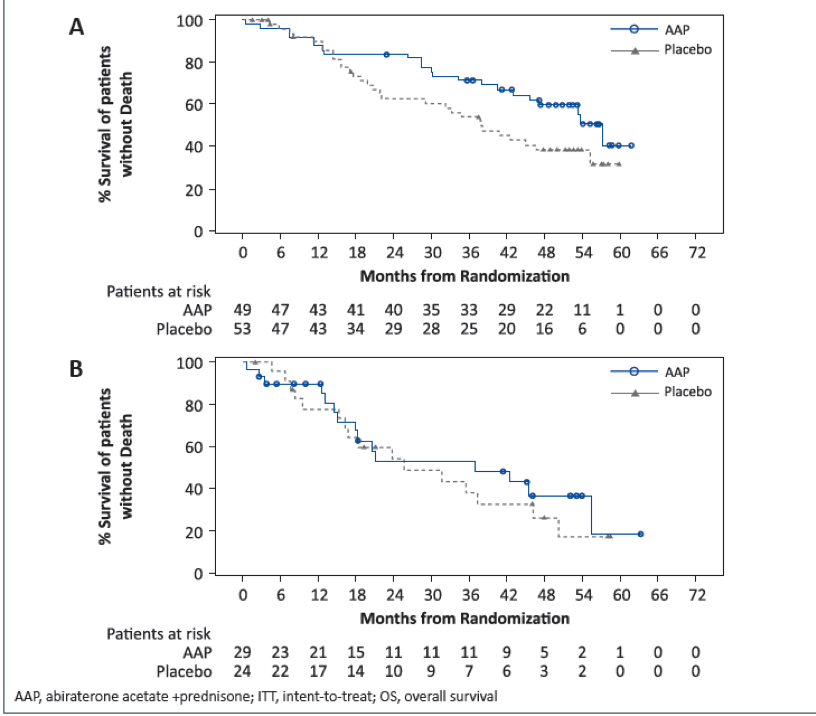
Figure 3 – Kaplan Meier plot of rPFS in lung and liver metastases:
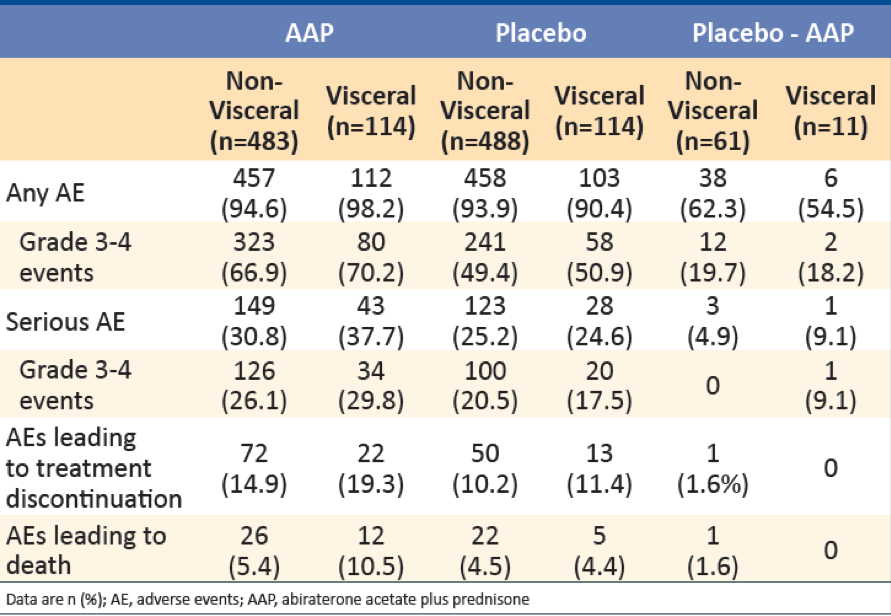
The overall incidence of adverse events (AEs) was similar in patients with visceral metastases (AAP: 98.2% vs. placebo 90.4%) and in patients without visceral metastases (AAP: 94.6% vs. placebo 93.9%). In patients with visceral metastases, Grade 3 or 4 serious AEs occurred in 34 (29.8%) patients in the AAP group and 20 (17.5%) patients in the placebo group. Treatment-related death in patients with visceral metastases was reported in one (0.9%) patient in each treatment group (AAP and Placebo) (Table 4).
Table 4 – Summary of adverse events:
In conclusion, AAP improved OS and rPFS in mHSPC patients with visceral disease, particularly in those with lung metastases. The presence of liver metastases was associated with a poorer prognosis than lung metastases. Interestingly, a benefit in rPFS or OS of AAP vs. placebo was not evident in patients with liver metastases, albeit the study is limited by the relatively small number of patients (n=53). The authors concluded that further exploration of the optimal treatment for men with prostate cancer with de novo liver metastases is required.
Presented by: Dr. Giulia Baciarello, Villejuif, CEDEX, France
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020.
References:
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. The Lancet Oncology 2019; 20(5): 686-700.


