For KEYNOTE-365, patients who failed or became intolerant to abiraterone after ≥4 weeks of treatment in the pre-chemotherapy mCRPC state and who progressed within 6 months of screening received pembrolizumab 200 mg IV Q3W plus enzalutamide 160 mg/day orally: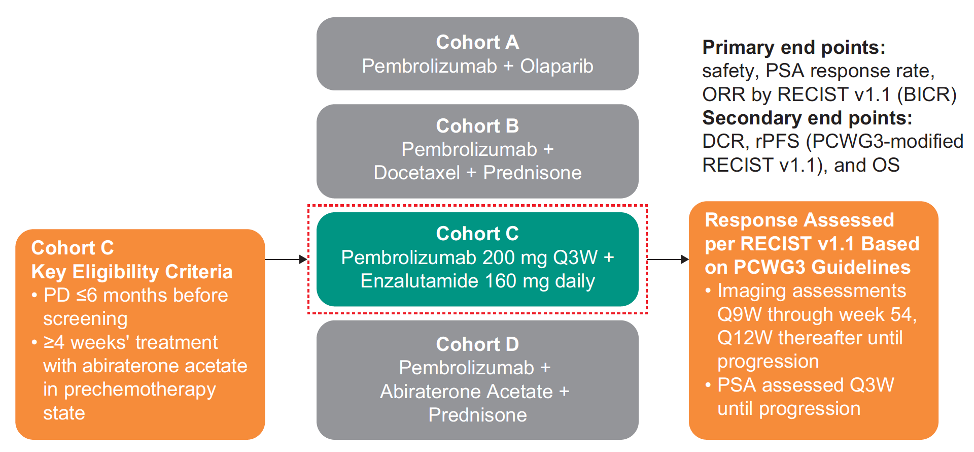
The primary endpoints were prostate-specific antigen (PSA) response rate (decrease ≥50% from baseline, confirmed by a second value ≥3 weeks later), ORR per RECIST v1.1 by blinded independent central review, and safety. Secondary endpoints included disease control rate (DCR), DOR, radiographic progression-free survival (rPFS) per PCWG-modified RECIST, overall survival (OS), time to symptomatic skeletal event, radiographic bone progression, and radiographic soft tissue progression. The database cutoff date was June 24, 2019.
There were 102 of 103 enrolled patients that were treated, including 39% who had measurable disease. The median time from enrollment to data cutoff was 19.1 months (range: 1.1-28.8) for all patients and 21.4 months (range: 15.1-28.8) for patients with ≥27 weeks of follow-up (n=69). Confirmed PSA response rate was 22% in 101 patients with a baseline PSA assessment. The PSA percentage change from baseline is as follows: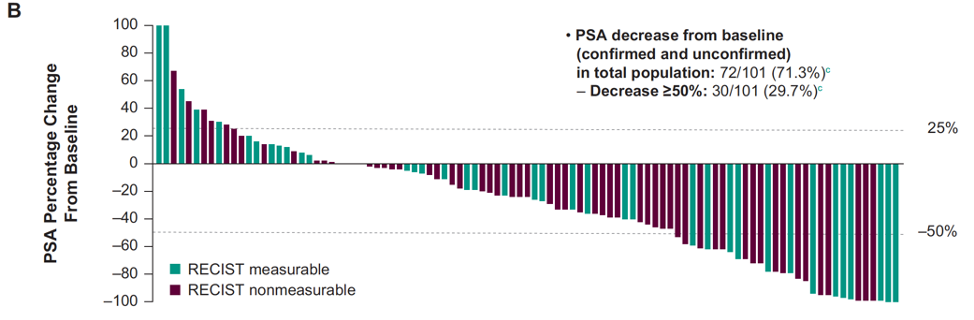
In patients with measurable disease and ≥ 27 weeks of follow-up (n=25), confirmed ORR was 12% (2 complete responses, 1 partial response):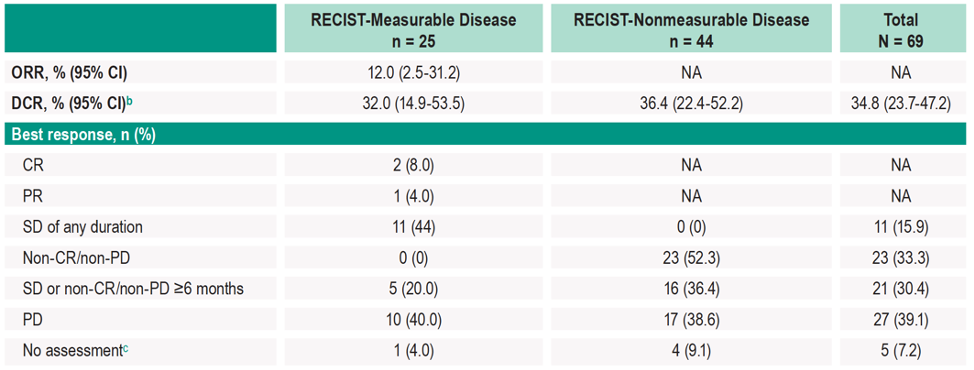
For all patients, median rPFS was 6.1 months (95% CI, 4.4-6.5) and median OS was 20.4 months (95% CI, 15.5-NR). At 12 months, the rPFS rate was 24.6% and OS rate was 72.8% by Kaplan-Meier.
The median time to radiographic bone progression was 8.3 months (95% CI 6.7-10.8):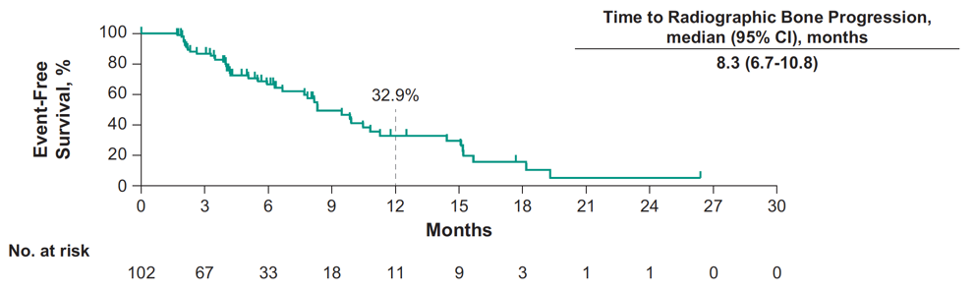
The median time to radiographic soft tissue progression was 15.2 months (95% CI 6.7 to not reached):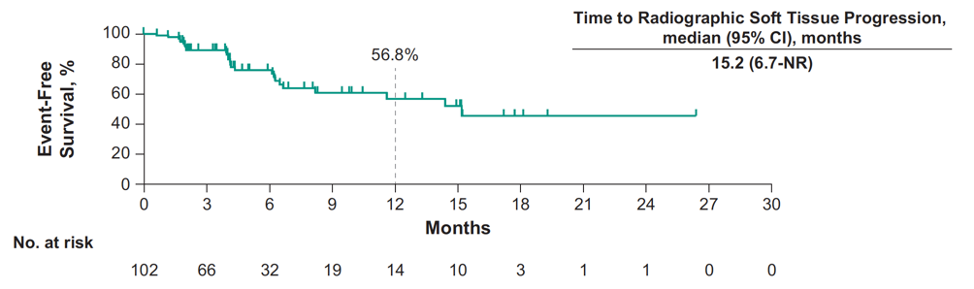
The median time to symptomatic skeletal-related event was not reached (95% CI 18.2 months to not reached):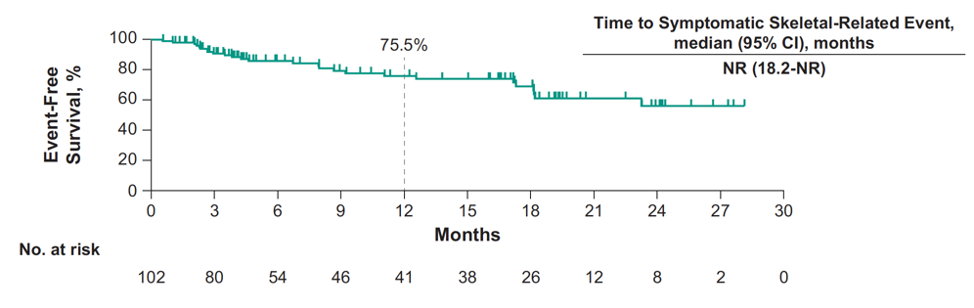
Grade ≥ 3 treatment-related adverse events occurred in 40 patients (39%), including 3 patients that died of adverse events, including one treatment-related.
Dr. Mourey concluded this updated analysis of cohort C of the KEYNOTE-365 with the following take-home messages:
- With increased enrollment and additional follow-up, pembrolizumab plus enzalutamide continued to show promising activity in patients with mCRPC previously treated with abiraterone acetate in the pre-chemotherapy state
- In general, the safety and tolerability of pembrolizumab plus enzalutamide was consistent with the individual profiles of each agent
- The promising data from this study support further evaluation of pembrolizumab plus enzalutamide in patients with mCRPC previously treated with abiraterone acetate
- A randomized phase 3 study of enzalutamide with or without pembrolizumab is open to enrollment of patients who are intolerant to or whose disease progressed on or after abiraterone acetate therapy for mCRPC and who did not receive chemotherapy for mCRPC (KEYNOTE-641, NCT03834493)
Presented by: Loic MOUREY, (FR), MD, Urology, Insitut Universitaire du Cancer–Oncopole, Toulouse, France
References:
- Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann Oncol. 2018 Aug 1;29(8):1807-1813
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol 2020 Feb 10;38(5):395-405.
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020


