In May 2020, the Food and Drug Administration (FDA) had approved two polyadenosine diphosphate–ribose polymerase inhibitors (PARPi) for the treatment of patients with mCRPC harboring (HRRm). Olaparib was approved based on the results of the PROfound trial3 for use in patients with mCRPC with BRCA1/2 and 12 other HRR gene alterations who progressed on prior therapy with either abiraterone and/or enzalutamide. It has also been included in the National Comprehensive Cancer Network (NCCN) Guidelines as a Category 1 recommended therapy.
Rucaparib, another PARPi, was approved based on the results of the open-label TRITON2 trial,4 for use in patients with mCRPC and BRCA1/2 alterations which have progressed on prior androgen receptor-targeted inhibitors and taxane chemotherapy. It has also been included in the NCCN Guidelines as a category 2A recommended therapy.
In the presented study, the authors aimed to examine the real-world treatment patterns of these patients in the pre-PAPRi era. The presented study was conducted in the United States prior to the PARPi approval, and it describes patient characteristics and real-world treatment patterns among patients with mCRPC and confirmed alterations in six HRR genes (BRCA1, BRCA2, ATM, CDK12, FANCA, and PALB2).
The study used the Flatiron Health Electronic Health Record (EHR)-derived database (flatiron.com/real-world-evidence/) from January 1, 2013, to March 31, 2019. This database contains longitudinal de-identified patient-level data from over 2 million active patients with cancer from over 800 oncology sites across the US. Approximately 90% of the Flatiron patients are followed in community oncology practices versus 10% in academic centers. The data included longitudinal information on patients’ cancer care (demographics, diagnoses, visits, labs, treatments, performance status) and cancer-related history.
Patients with molecular tests for selected HRRm gene alterations were identified, and the test result was extracted. Of 5,213 adult prostate cancer patients with a confirmed mCRPC diagnosis, and at least one month of follow-up post-mCRPC diagnosis, 674 underwent molecular testing for gene alterations in BRCA1, BRCA2, ATM, CDK12, FANCA, and PALB2 during the study period (before or after the mCRPC diagnosis).
A total of 160 patients tested positive for at least one of these six HRR gene alterations and were included in the HRRm+ mCRPC sample. This was a retrospective longitudinal observational cohort study. The date of mCRPC diagnosis was defined as the latter between the date of first metastasis and the date of first CRPC diagnosis. Patients were followed from the mCRPC diagnosis date to the end of observed clinical activity or death. All patients had at least one month of follow-up post-index by design.
Treatment patterns were assessed post-mCRPC diagnosis and included the line of therapy (LOT) sequences post-mCRPC diagnosis by treatment class, regimens by LOT (class and agent-level), as well as the median months on and off treatment for LOTs by class.
Prostate cancer treatments post-mCRPC diagnosis were classified according to five classes of therapy:
- Next-generation hormonal agents (NHAs; i.e., abiraterone, enzalutamide, apalutamide, and darolutamide)
- Chemotherapy (docetaxel, cabazitaxel)
- Immune-oncology (sipuleucel-t, thalidomide, BCG vaccine, nivolumab, atezolizumab, lenalidomide, durvalumab, and ipilimumab)
- Other (i.e., pembrolizumab, targeted therapies, radium-223, and clinical study drugs; did not include non-NHA hormonal therapies or bone therapy agents)
- Combinations of the four classes above
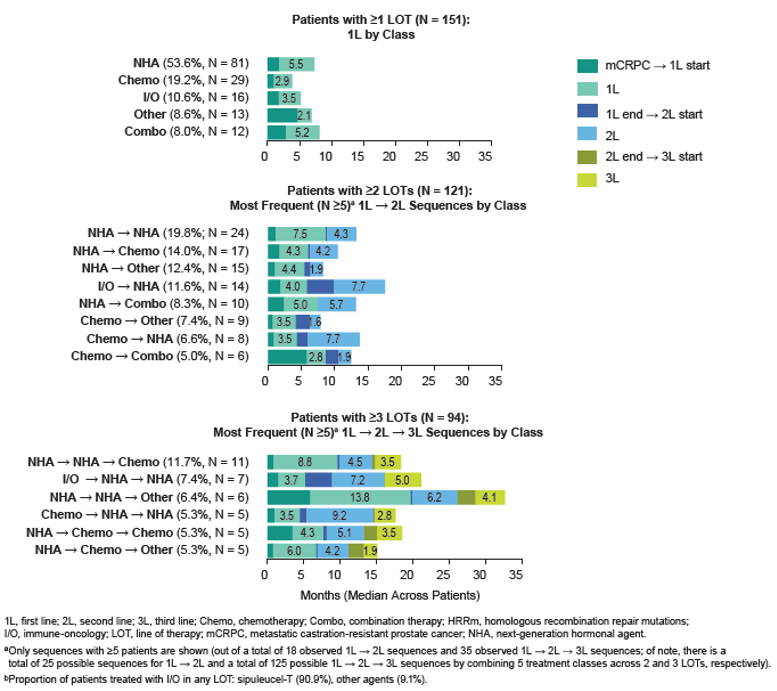
Among 151 HRRm patients for whom ≥1 LOT was observed post-mCRPC diagnosis, 121 (80.1%) were treated with ≥ 2 LOTs, and 94 (62.3%) were treated with ≥3 LOTs during follow-up (Figure 1). Approximately a third of deaths occurred after only 1 or 2 LOTs: among the 63 patients for whom death was observed during the study period, 10 (15.9%) died after 1 LOT, 9 (14.3%) died after 2 LOTs, 15 (23.8%) died after 3 LOTs, 7 (11.1%) died after 4 LOTs, and 22 (34.9%) died after ≥5 LOTs. Multiple distinct LOT sequences were observed (Figure 2).
Table 1 – Baseline characteristics of HRRm mCRPC patients:
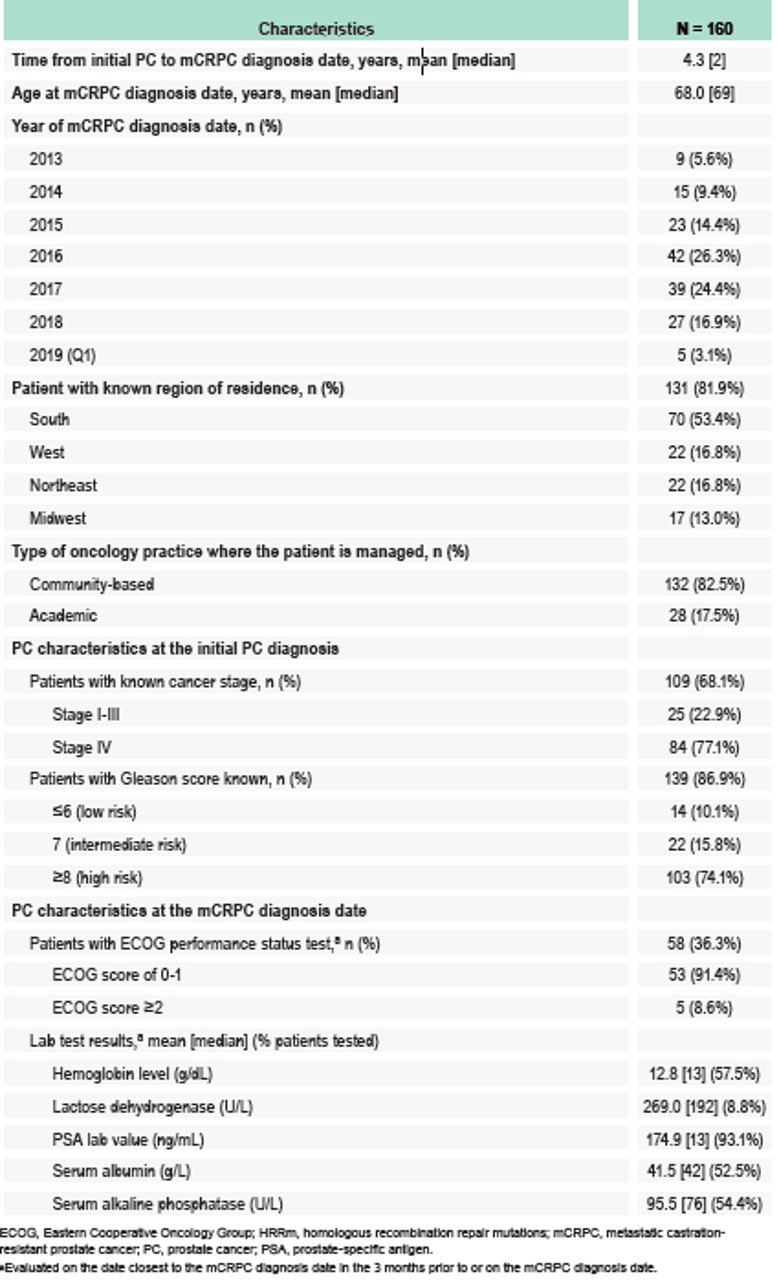
Figure 1 – mCRPC treatment patterns:
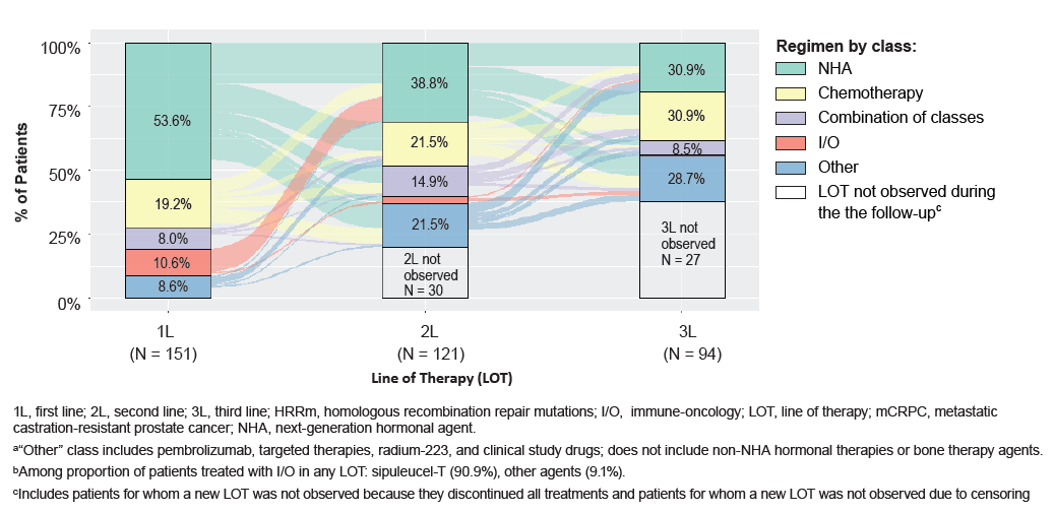
Figure 2 – The most frequent line of therapy sequences:
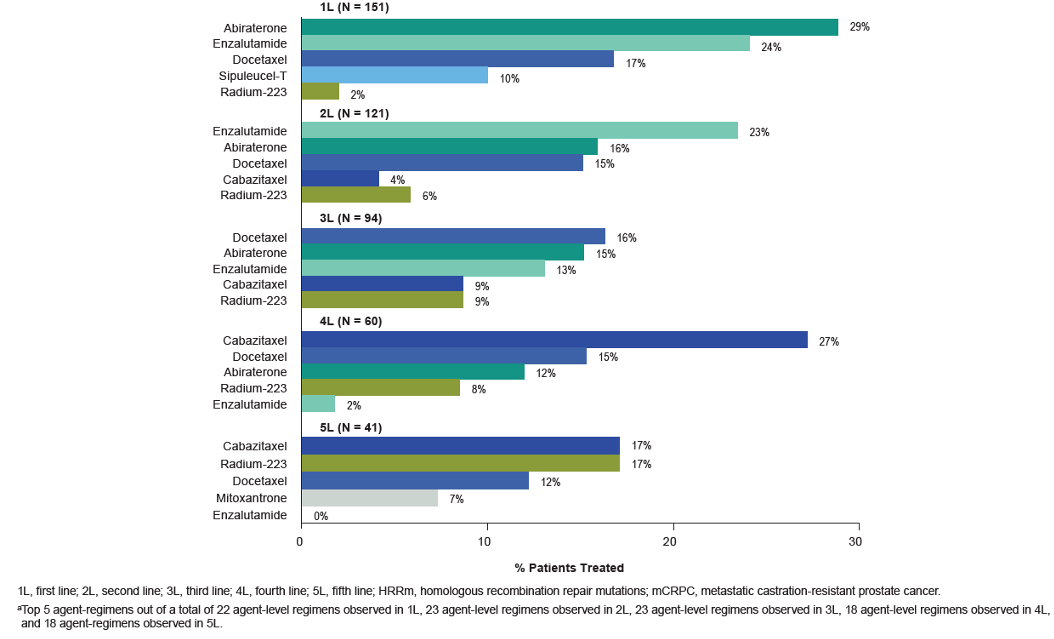
The top five most common treatment regimens are shown in figure 3.
Figure 3 – Top five Most Common HRRm mCRPC Regimens (Agent Level) by Lines of Therapy:
The authors report several limitations to their study:
- Flatiron Health data are mostly drawn from community oncology practices and thus may not be representative of treatment patterns at academic centers
- Given that the study was conducted in the pre-PARPi era, patients were referred to HRRm testing to guide genetic counseling or inclusion in clinical trials; therefore, they may not be representative to the full population of HRRm mCRPC patients
- The number of patients in the study sample was low and may not be representative of all patients with mCRPC and HRRm
- LOTs were inferred from patterns of treatment administrations/orders as recorded in the EMR data; occasional misclassification of LOTs may have occurred
- Time on treatment is reported as the median across all patients; however, some regimens/LOTs have higher individual variability than others in time on treatment
- The choice of therapy and treatment duration may have been influenced by patient characteristics (e.g., fitness level, response to prior treatment), which were not available in the database and, therefore, could not be controlled for in the analysis
With the approval of PARPis, more treatment options are now readily available for patients with HRRm mCRPC. However, it is imperative that early genomic testing and identification of HRRm alterations are performed to ensure that HRRm patients benefit from currently available life-prolonging treatment with PARPis.
Presented by: Neal Shore, MD, FACS, is the Medical Director of the Carolina Urologic Research Center. He practices with Atlantic Urology Clinics in Myrtle Beach, South Carolina
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020
References:
1. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015; 161(5): 1215-28.
2. Mateo J, Boysen G, Barbieri CE, et al. DNA Repair in Prostate Cancer: Biology and Clinical Implications. European urology 2017; 71(3): 417-25.
3. de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine 2020; 382(22): 2091-102.
4. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients (pts) with DNA damage repair (DDR)-deficient metastatic castration-resistant prostate cancer (mCRPC): Updated analyses. Annals of Oncology 2019; 30: v327-v8.


