(UroToday.com) The POLE gene is one of the components of DNA polymerase epsilon, which is responsible for the replication of the leading DNA strand. POLE contains a DNA polymerase domain, as well as an exonuclease domain responsible for proofreading replication errors.

Inactivating mutations in the POLE exonuclease domain are rare, but when present impair its proofreading capability, resulting in high rates of missense and nonsense mutation and overall high mutational burden (often greater than 100 mutations/Mb). Not all mutations in POLE are pathogenic, as illustrated in the figure below. Patients with germline or somatic mutations in POLE usually have intact DNA mismatch repair mechanisms.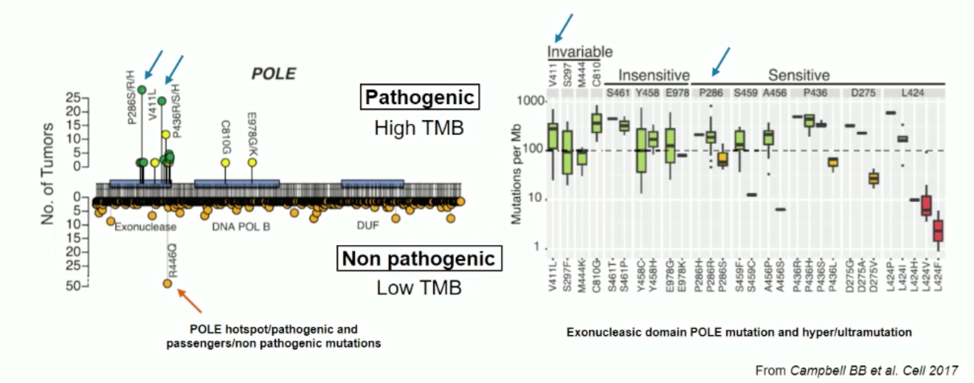
Given the associations between POLE mutation, high tumor mutational burden, and a potentially higher likelihood of response to immune checkpoint blockade, the authors hypothesized the nivolumab may have efficacy in POLE-mutated tumors. The following clinical trial schema was designed to pursue this question.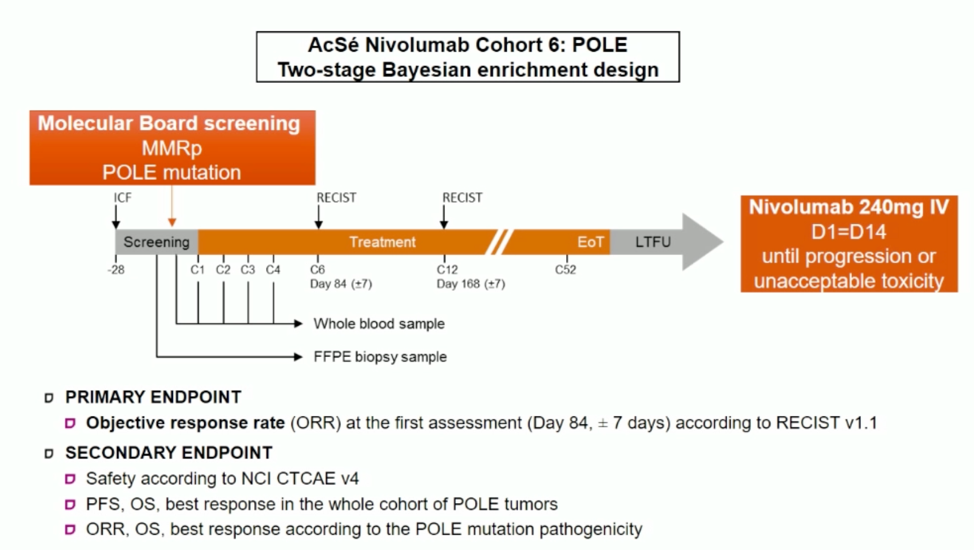
Patients with advanced (unresectable or metastatic) malignancies with measurable disease that is refractory to standard therapies were eligible for this trial if they were found to have proficient DNA mismatch repair and POLE missense mutations within or close to the exonuclease domain. The likely pathogenicity of these mutations was assessed by an independent molecular tumor board that was blinded to the tumor mutational burden.
The two-stage Bayesian enrichment trial design is as follows: Ten patients were included in the initial stage, and if overall response criteria were met, then the study would proceed in 5 patient intervals, and continue based on the assessment of the association between POLE mutations and outcome. Since the initiation of the trial in 2018, 50 patients have been screened and 16 patients enrolled. Patient characteristics and POLE mutation presumed pathogenicity are shown below.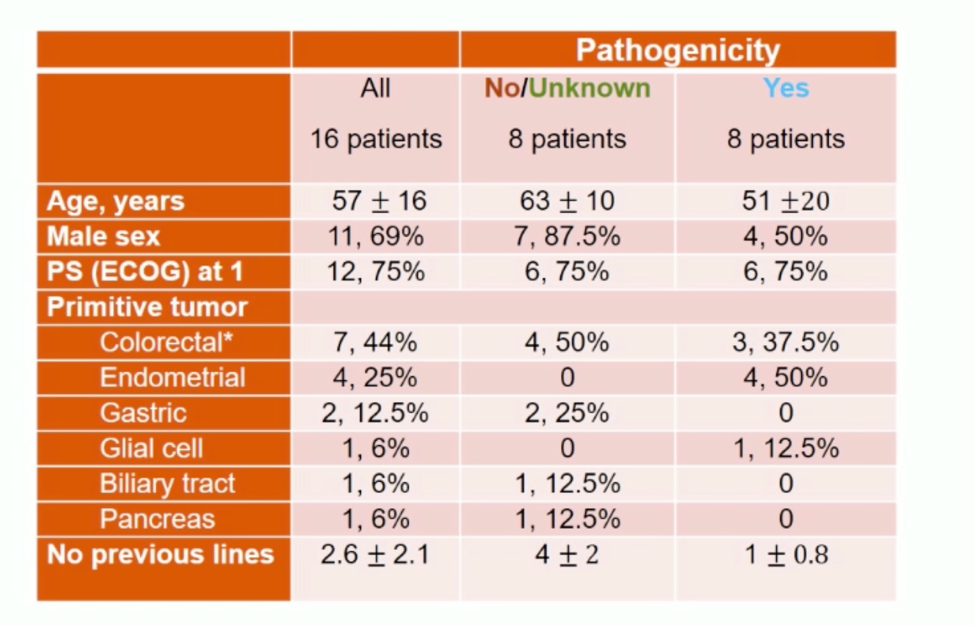
Toxicities observed were in line with prior reports of nivolumab safety and included 1 case of grade 3 nephritis resulting in discontinuation, 1 case of grade 2 diffuse pulmonary interstitial disease requiring treatment pause. Two patients died due to overall disease progression, not felt to be related to treatment.
Efficacy data are shown below. Responses were mixed, though it is notable that all colorectal cancer patients in this study had at least stable disease (1 patient) or response (5 patients).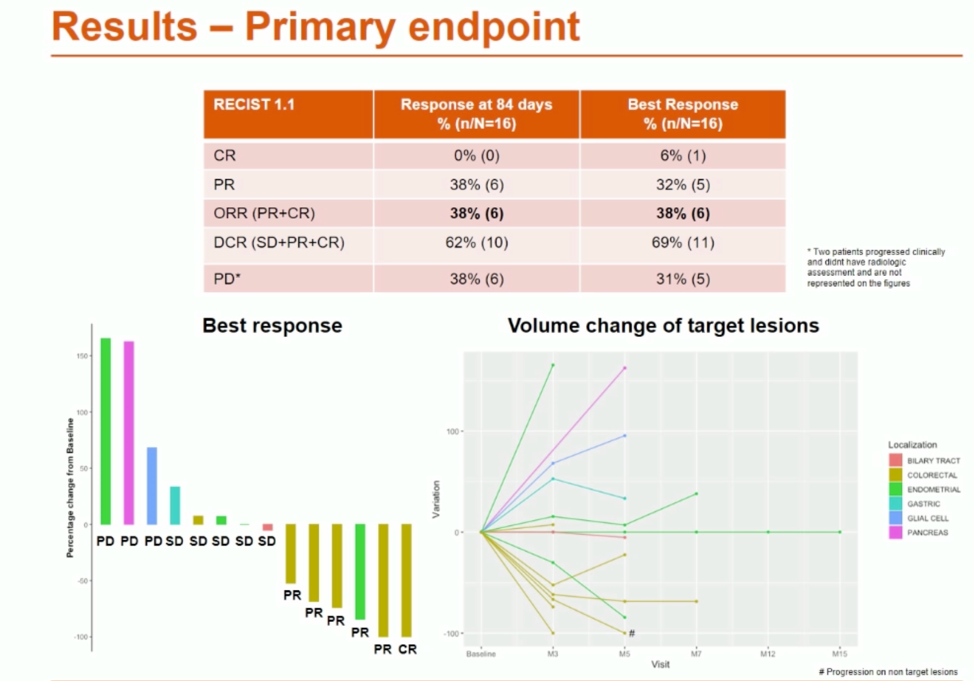
Importantly, responses were enriched in patients with known pathogenic mutations in POLE.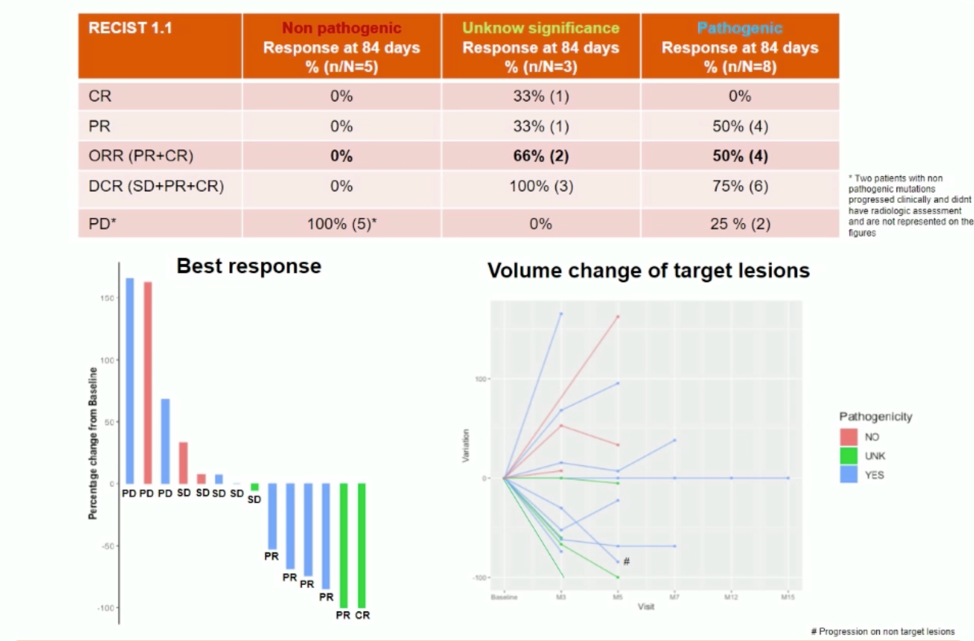
Though overall patient numbers are small, there is a suggestion of improved progression-free survival (PFS) and overall survival (OS) benefit with nivolumab therapy in patients with pathogenic POLE mutation relative to non-pathogenic mutations. Within this patient cohort, the presented results suggest that tumors with pathogenic or likely pathogenic POLE mutations and intact mismatch repair (especially colon cancers) may benefit from nivolumab therapy relative to tumors without these mutations.
Presented by: Benoit Rousseau, MD, PhD, Solid Tumor Division, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.


