In this presentation, Dr. Ben Tran shared results from the dose-escalation phase of AMG 160 study, a half-life extended (due to Fc fragment characteristics) prostate-specific membrane antigen (PSMA) and CD3 BiTE, in patients with mCRPC (NCT03792841). In preclinical experiments, AMG 160 induced potent and selective killing of PSMA positive cells in vitro and regression of tumors in vivo, supporting its advancement into clinical testing. A schema showing the mechanism of action of this BiTE is shown below.

The schema of the presented clinical trial is shown below. In Part 1, which is a first in-human study of AMG 160 monotherapy, doses were administered in an escalating manner to assess safety, tolerability, and identify a recommended phase 2 dose. Part 2 will be a trial in combination with pembrolizumab. Due to the half-life extended nature of the BiTE, it is able to be administered IV every 2 weeks.

Key inclusion and exclusion criteria are shown below. Importantly, patients who received prior PSMA radionuclide therapy such as lutetium were eligible for inclusion. At the data cutoff, 43 patients had received 1 or more doses of AMG 160. The majority of patients had received 4 or more prior lines of therapy.

Treatment-emergent and treatment-related adverse events were noted in almost all patients (95%). No patients had adverse events that resulted in treatment discontinuation. The most common adverse events are illustrated in the table below, with the most common being cytokine release syndrome. Six patients assessed developed anti-drug antibodies that affected drug exposure while on treatment.

Cytokine release syndrome (CRS) was most severe in cycle 1. Presentation varied, most commonly associated with fever, hypotension, nausea, vomiting, diarrhea, and transient transaminitis. 11 patients had grade 3 CRS, and 6 patients had hypotension requiring pressor support. Transaminitis events were reversible. No patients had grade 4/5 CRS events and no patients required treatment discontinuation due to CRS.
To mitigate CRS in later cohorts within this study, several strategies were pursued including dose priming, dexamethasone premedication, and prophylactic IV hydration. This optimized mitigation strategy seemed to be effective, reducing serious adverse events and preventing grade 3 CRS, as shown below.

Efficacy data is shown below. 8 patients had prostate-specific antigen (PSA) reductions of more than 30%. For the 13 patients that had circulating tumor cell numbers assessed, 3 patients had no CTCs detected after treatment. 8 patients had stable disease; 2 patients had partial responses. The swimmer’s plot below illustrates the clinical trajectory of patients, stratified by treatment cohort. Six patients are still continuing on therapy past 6 months.

PSA responses appear to be dose-dependent as shown in the figure below with the treatment cohort denoted by bar plot color. Two patients, indicated with triangles, had PSA responses despite having previously progressed on lutetium-PSMA treatment.

Dr. Tran then showed three examples of patient responses with available imaging and PSA response kinetics.
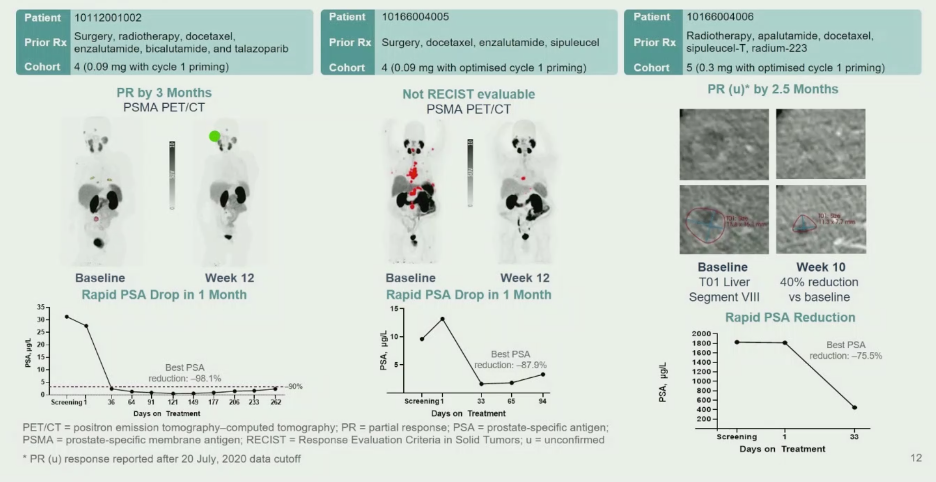
In conclusion, AMG 160 as monotherapy was felt to have a manageable safety profile. The most common side effect was cytokine release syndrome, which could be mitigated with priming doses, steroids, and hydration. 44% of patients were able to stay on therapy at the time of data analysis, with 6 patients receiving therapy for more than six months. Despite multiple prior lines of therapies, patients showed evidence of response by PSA decline (34.4% with more than 50% decline) and imaging (of 15 patients with measurable disease, 2 confirmed partial responses, 1 unconfirmed partial response, 8 stable disease). The impact of steroid administration and the development of anti-drug antibodies on AMG 160 efficacy is unclear at this point. Overall, this strategy appears to represent a tolerable and exciting therapeutic modality for men with mCRPC that warrants further investigation.
Presented by: Ben Tran, MBBS, FRACP, Consultant Medical Oncologist at the Peter MacCallum Cancer Centre, Melbourne, Australia
Written by: Alok Tewari, MD, Ph.D., Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
Related Content:
Interim Results from AMG 160 a half-life extended (HLE), PSMA-targeted, bispecific T-cell engager (BiTE®) immune therapy for metastatic castration-resistant prostate cancer (mCRPC) - Ben Tran


