Patients with castration-resistant prostate cancer (CRPC) (metastatic or nonmetastatic) were eligible if they had castrate levels of testosterone, had received chemotherapy more than 1 year prior for CRPC, or more than 3 months prior if castration-sensitive disease at that time. The primary endpoint was objective response rate or PSA decline greater than 30% sustained for more than 21 days. Progression was defined as radiographic progression or PSA increase of over 1 ng/mL confirmed by two values a week apart.
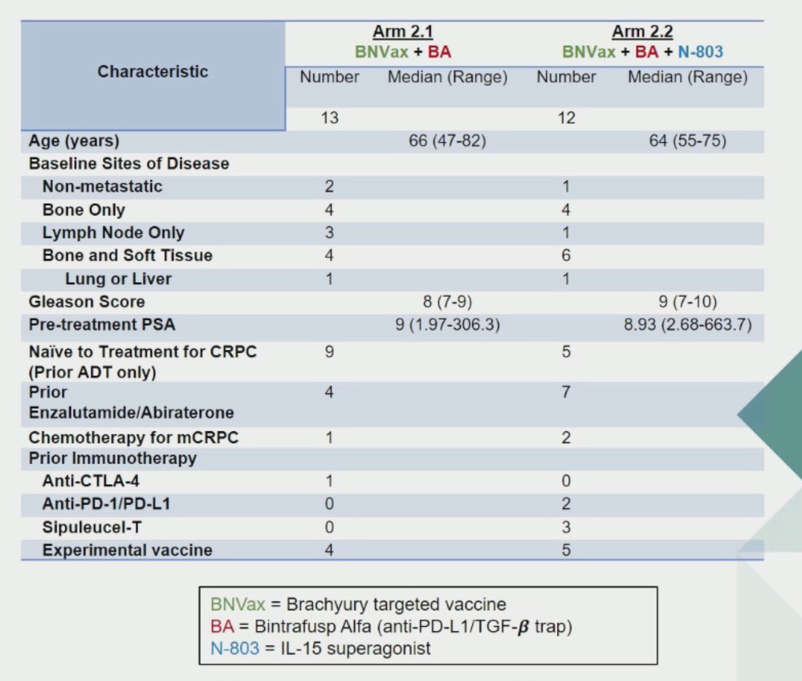
In the first arm of the study, CRPC patients were first treated with BNVax and bintrafusp alpha. After safety was established, a second arm of patients were treated with all agents. Patient demographics are shown below.
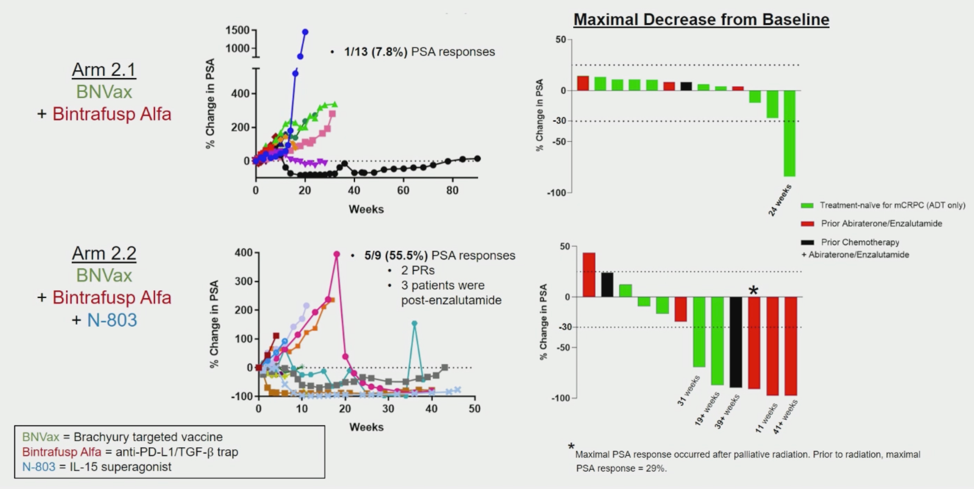
In the first arm of the BNVax plus bintrafusp, only 1 patient had a sustained prostate-specific antigen (PSA) response. The addition of N-803 resulted in 5 patients having a sustained PSA response and two of those patients also had a radiographic response.
Most side effects were grade 1-2, including transient fever, chills and injection site reactions. Three patients in each arm had grade 3 adverse events (tumor hemorrhage, ACTH deficiency, pancreatitis, diabetes, cystitis and eosinophilia). No grade 4 events were observed. Of note, all four patients who developed ACTH deficiency (of any grade) were PSA responders.
In summary, this triple immunotherapy combination produced a PSA response rate of 55%, including 2 radiographic partial responses in patients who had progressed on standard of care anti-androgen therapy. The significance of isolated ACTH deficiency in PSA responders is unclear and requires further investigation.
Presented by: Jason M. Redman, MD, Assistant Research Physician, Cancer Immunotherapy Program, Genitourinary Malignancy Branch, Medical Oncology Service, National Cancer Institute, Bethesda, MD
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.


