VERU-111 is an inhibitor of microtubule polymerization that is not a substrate for multidrug resistance proteins or the CYP3A4 system. Pre-clinical studies have shown that VERU-111 can induce apoptosis in mCRPC prostate cancer cell line models that are resistant to taxane and enzalutamide. This clinical study was a dose-escalation trial in mCRPC patients who had to have received 1 prior anti-androgen therapy. Prior taxane administration was allowed. Across 7 centers, 39 patients were enrolled. A two-part dosing strategy was utilized using a 3x3 dose-escalation strategy. In the first part, patients took VERU-111 daily for days 1-7 then had 14 days off. If tolerated, in the second part, patients started with drug dosing on days 1-14 with 7 days off, then if safe, would take daily until toxicity or disease progression. The primary outcome of the study was safety.
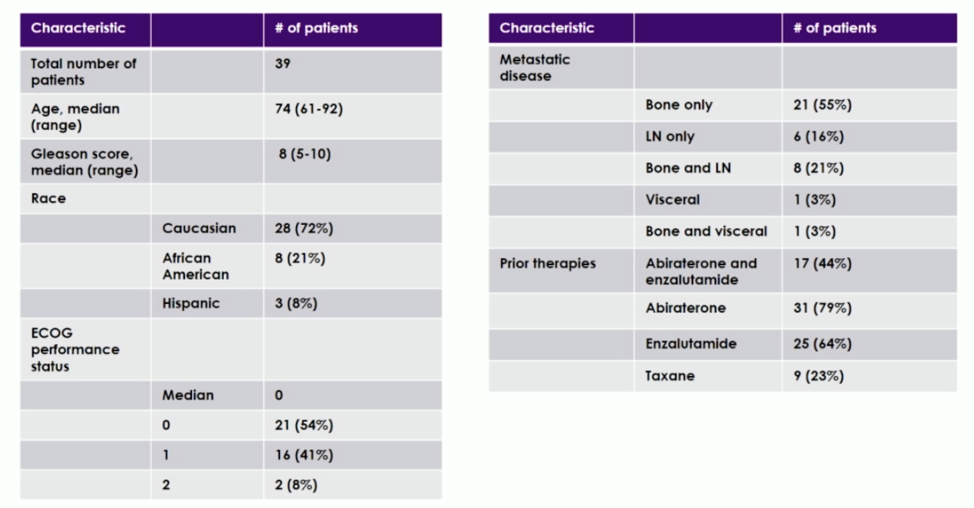
Patient demographics are shown below. The median age was 74. The majority of patients had bone-only metastatic disease, and 9 patients had previously received taxane.
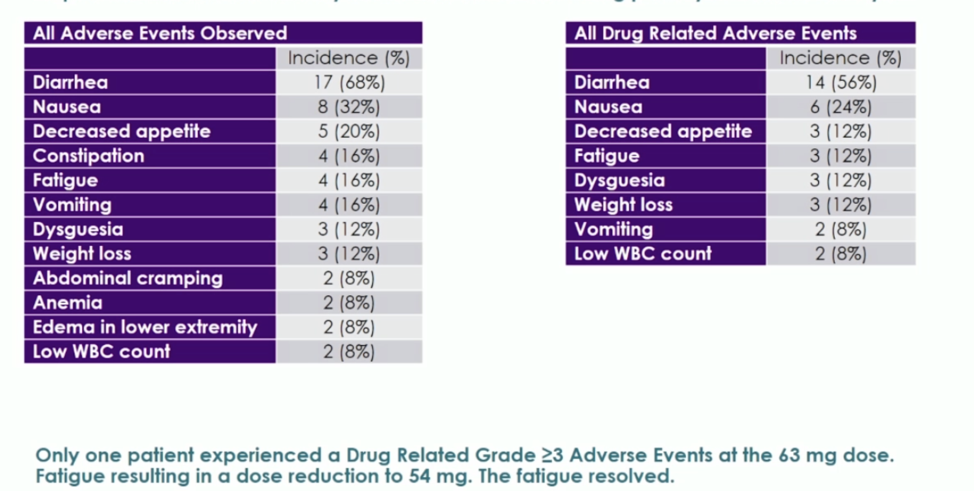
The most common toxicities were diarrhea, fatigue, and nausea. The maximum tolerated dose was 72 mg, but given the incidence of diarrhea at this level, the RP2D 63 mg. The toxicity at the 63 mg dose is shown below. Notably, at this dose, no grade 3 diarrhea, neurotoxicity, or neutropenia was observed. All drug-related adverse events shown below were grade 1-2.
Ten men were able to progress through dose escalation to receive at least 4 cycles of 21-day dosing. Two of these patients had partial responses, and eight had stable disease. Two of ten patients had a greater than 50% decline in their PSA. The median time to radiographic progression in these patients was 11 months, and five of the ten men are continuing on treatment.
A single-arm open-label phase 2 study of VERU-111 at the dose of 63 mg is ongoing. This will recruit a total of 40 patients with mCRPC who have received at least 1 prior anti-androgen therapy, but taxane prior therapy will not be allowed. The primary endpoint will be radiographic progression-free survival.
Presented by: Mark C. Markowski, MD, Assistant Professor of Oncology, The Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.


