She began by highlighting the overall success of STAMPEDE, calling it the “most innovative and unprecedented trial design” in prostate cancer therapeutics. She highlighted that data from this trial has benefits patients worldwide and established a number of new approaches as standard of care including ADT plus docetaxel, ADT plus local radiotherapy, and ADT plus abiraterone acetate and prednisolone in patients with advanced prostate cancer.
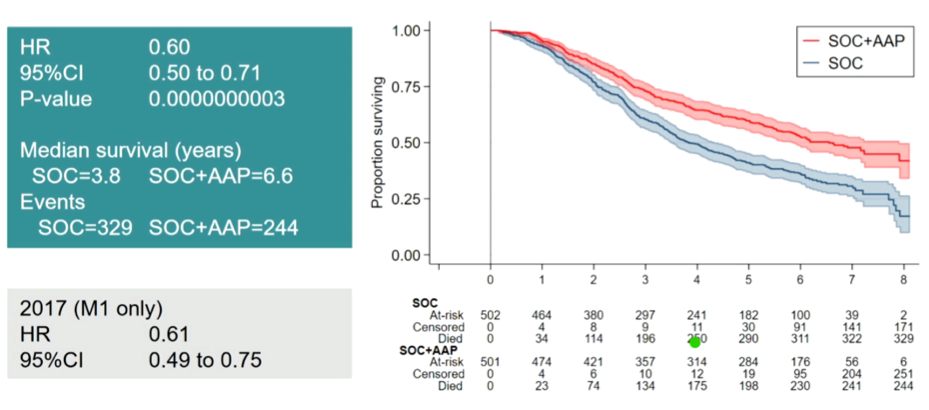
She began highlighting the data presented by Dr. James confirming the overall survival benefit of abiraterone acetate and prednisolone in addition to ADT seen with prolonged follow-up.
She then contextualized these data among the various other treatment options for patients with metastatic hormone-sensitive prostate cancer. She emphasized the similarity of effect for abiraterone acetate seen between STAMPEDE and LATITUDE.
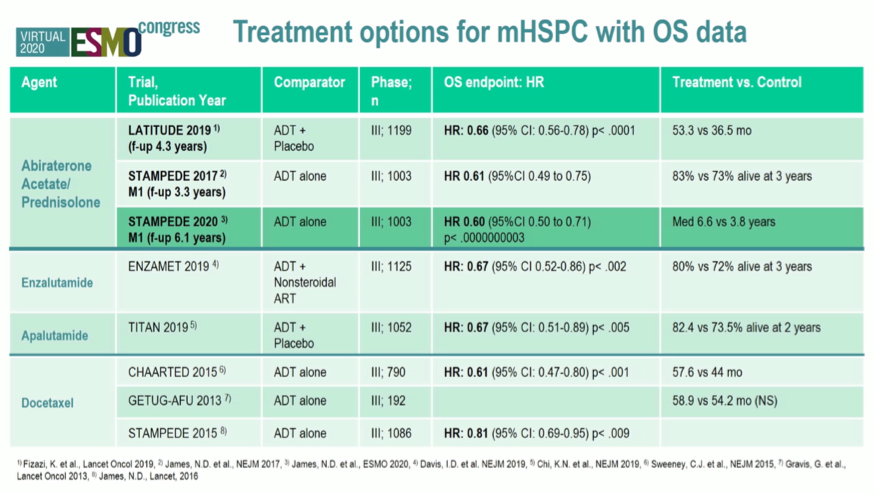
Dr. De Santis emphasized that STAMPEDE includes a wider spectrum of disease characteristics than was included in LATITUDE. Notably, the data presented by Dr. James confirmed the benefit of abiraterone acetate inconsistent across disease risk and volume strata.
She highlighted that the generally favourable safety profile of abiraterone acetate seen in this analysis inconsistent with data from the COU-302 trial.
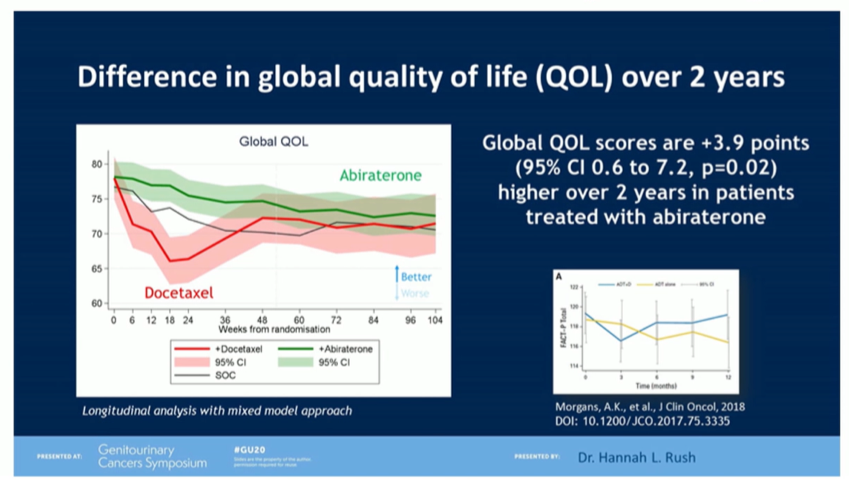
She then highlighted a recent quality of life analysis between abiraterone acetate and docetaxel in men with mHSPC. This analysis suggested that particularly within the first year following the start of treatment, quality of life was better for those receiving abiraterone as compared to docetaxel.
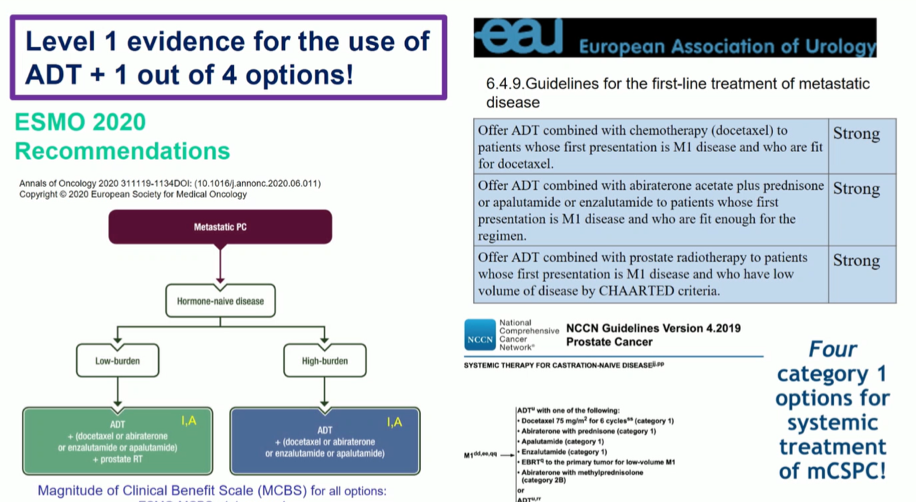
Dr. De Santis then transitioned to a discussion of the current guideline environment. There is evidence supporting the use of four different agents in addition to ADT in patients with mHSPC. These approaches are widely recommended across many guideline bodies.
She then highlighted recently presented data from Dr. Neal Shore in which 81% of patients treated in the United States did not receive one of these guideline-recommended agents. Similarly, in the United Kingdom, a recent audit suggested that only 1 in 4 men with advanced prostate cancer received life-prolonging chemotherapy, despite recommendation from the National Institute for Health and Care Excellence (NICE).
Particularly in the context in the COVID-19 pandemic, the use of abiraterone acetate may be preferred to docetaxel for a variety of reasons. However, NICE has yet to approve abiraterone in this disease space.
With respect to treatment selection, Dr. De Santis highlighted the role of expert opinion as discussed at the APCCC which demonstrated no consensus as of the 2019 meeting with respect to which agent to use in mHSPC though 85% felt that some form of additional therapy should be given along with ADT. Today, we derive treatment decisions on the base of clinical features but Dr. De Santis highlighted that molecular profiling may be helpful in the future. Interestingly, there were minimal differences in genomic profiles on the basis of tumor volume. However, currently, this is not ready for clinical use in mHSPC, unlike mCRPC.
Concluded, Dr. De Santis suggested that this long term follow-up of STAMPEDE Arm G supports the role of abiraterone acetate in this disease space.
Presented by: Maria De Santis, MD, Medical Oncologist and Chair of Section for Interdisciplinary Genito-Urinary Cancer Medicine, Charité Medical University Hospital, Berlin, Germany.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center @WallisCJD on Twitter at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
Related Content:
ESMO Virtual Congress 2020: Abiraterone Acetate Plus Prednisolone for Hormone-Naïve Prostate Cancer: Long-Term Results from Metastatic Patients in the STAMPEDE Randomized Trial


