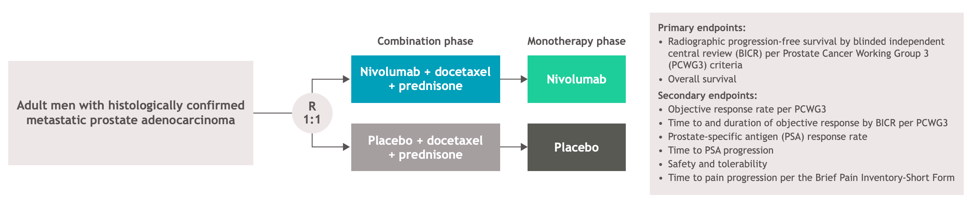
The schema of the trial is shown below:
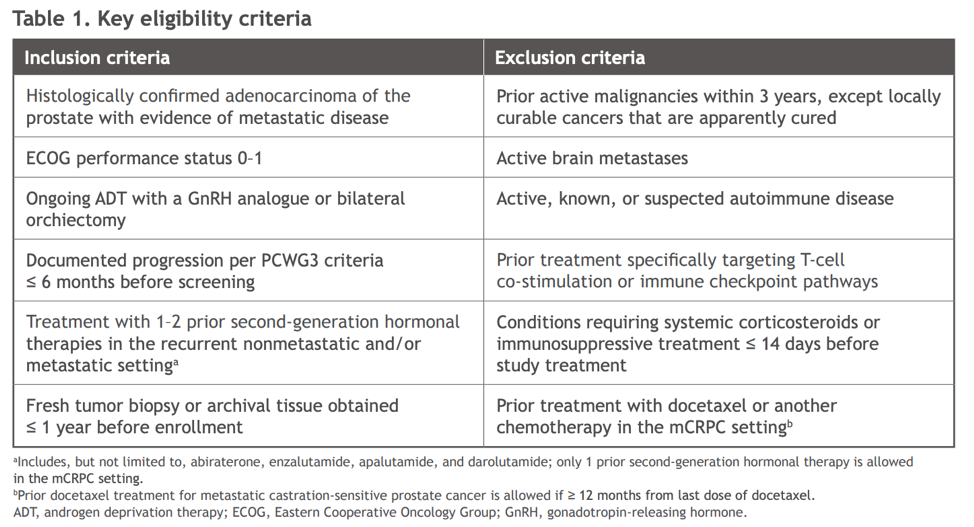
Key eligibility criteria are shown below:
Target enrollment is 984 patients. Study sites are currently open in the United States, Australia, Asia (Japan, Republic of Korea, Singapore), and Europe (Austria, Belgium, Czech Republic, France, Poland, Spain and the United Kingdom).
Presented by: Charles G. Drake, MD, PhD, Professor of Oncology, Urology and Immunology, Director of Genitourinary Oncology, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, Boston, MA, 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
Reference:
- Fizazi K., Gonzalez Mella P., Castellano D. "Efficacy and safety of nivolumab in combination with docetaxel in men with metastatic castration-resistant prostate cancer in CheckMate 9KD" Annals of Oncology. Volume 30, Supplement 5 October 2019. https://doi.org/10.1093/annonc/mdz394.045


