In an attempt to provide expert guidance on the treatment of patients with advanced prostate cancer, Dr. Silke Gillessen has convened the Advanced Prostate Cancer Consensus Conference (APCCC). Recognized global clinical experts who treat a high volume of patients with prostate cancer and engage in research and education gather for the APCCC and, thus, the consensus statements that result from the APCCC meeting reflect the opinions of theses thought leaders.
In a poster presentation at this year’s European Society of Medical Oncology (ESMO) 2020 Virtual Annual Meeting, Dr. Ryan assessed the hypothesis that non-panelists (that is, clinicians who treat prostate cancer who were not part of the APCCC consensus) would differ in their opinions from those expert panelists.
To assess this, 20 representative questions posed at APCCC2019 were posted online on UroToday.com between 9/8/2019 and 8/1/2020, prior to public dissemination of the APCCC consensus results. Engagement with these questions was solicited via email to several national societies and groups.
Clinicians included in the “non-panelist” group treated between 10 and 100 prostate cancer patients annually and the authors assessed the concordance between the most frequently chosen answers among APCCC panelists and the electronically surveyed non-panelists.
Medical oncology was the most heavily represented specialty among both APCCC panelists (44%) and non-panelists (52%). Urologists were more heavily represented among non-panelists (38%) than APCCC panelists (30%).
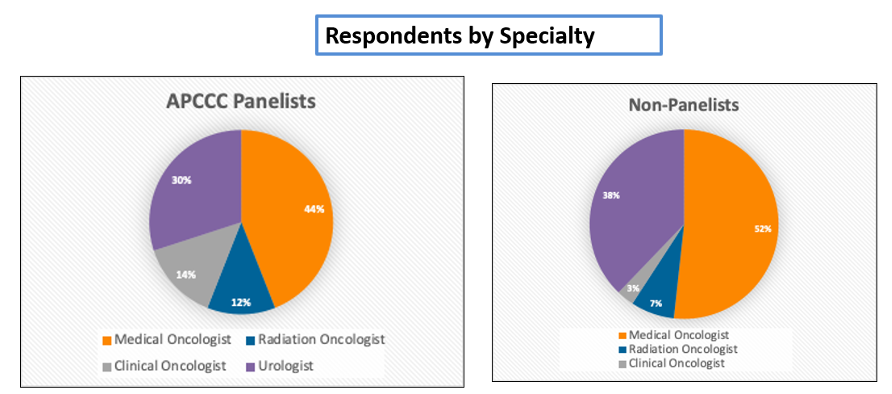
Responses from non-panelists were more geographically diverse than those from panelists.
Notably, for 12 of 20 analyzed questions (60%), there was an agreement between APCCC panelists and non-panelist respondents while 8/20 questions had significant differences between APCCC panelists and non-panelist respondents. The questions with the greatest discrepancies were:
1. “Do you recommend genetic counseling and/or germline DNA testing for patients with newly diagnosed metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC)?” for which affirmative responses were much more common amongst APCCC panelists.
2. “Based on the current literature, do you think that local treatment of the primary tumor has an overall survival benefit in patients with newly diagnosed metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC)?” for which APCCC panelists which much more likely to state that this benefit was present, but only among those patients with a low-volume of disease.
3. “Do you recommend anti-PD1 therapy for patients with metastatic prostate cancer and a mismatch repair defect (MSI high) outside of a clinical trial?” for which APCCC panelists were much more likely to utilize this approach, and use it earlier in the disease process.
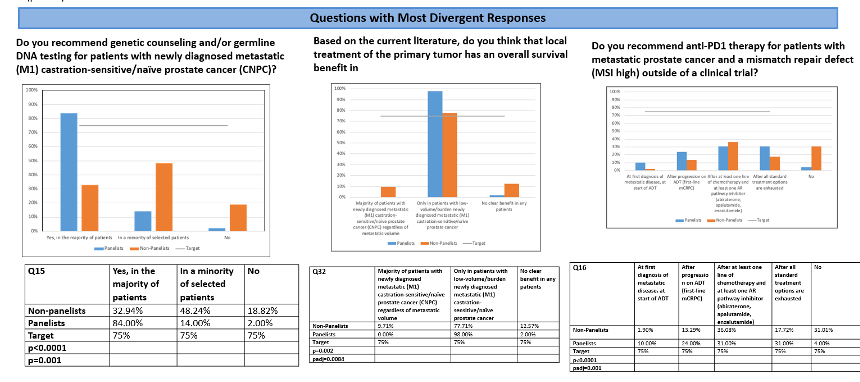
Other questions with divergent responses included:
1. “Do you recommend Lutetium-PSMA therapy for patients with PSMA imaging-positive mCPRC who have exhausted approved treatments and cannot enroll in a clinical trial?” (P=0.004)
2. “Do you recommend switching treatment for mCRPC at PSA progression alone (in the absence of other examinations)?” (P=0.02)
3. “What is your preferred treatment recommendation in the majority of patients with newly diagnosed cN1 (pelvic lymph nodes), M0 prostate cancer?” (p=0.03)
4. “What is your preferred treatment in addition to ADT in patients with newly diagnosed low-volume metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC) relapsing after local treatment of the primary tumor?” (P=0.005)
5. “What is your preferred treatment in addition to ADT in patients with newly diagnosed high volume metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC) relapsing after local treatment of the primary tumor?” (P=0.03)
The authors concluded that, in spite of a preponderance of agreement between expert APCCC panelists and non-panelist respondents to the same questions, there were notable arms of discrepancies which, for the most part, reflect topics in which there is relatively recent and sometimes controversial data.
Presented by: Charles J. Ryan, MD is the B.J. Kennedy Chair in Clinical Medical Oncology at the University of Minnesota and Director of the Division of Hematology, Oncology and Transplantation
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.


