In total, 150 patients were identified for analysis. Their demographics are shown below.
The median overall survival of patients in this cohort was 14.5 months, consistent with the ALSYMPCA results. Based on univariate and multivariate analyses, the authors identified four baseline variables: ECOG performance status, alkaline phosphatase level, PSA level, and albumin level as independently associated with survival. Using these factors, they created as prognostic index model score that stratified patients into risk groups that were associated with survival, as seen below.

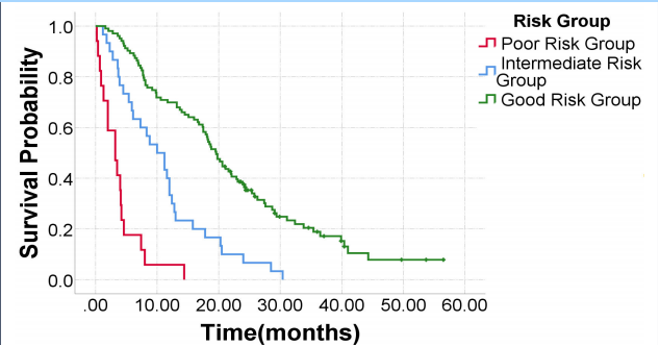
The prognostic index model had an AUC of 0.762. The authors identified several post-treatment factors as significantly associated with survival with Ra-223 therapy as well. Receiving 4 or more doses of radium, hematologic toxicity, receiving systemic therapy after Ra-223, and PSA responses due to therapy were associated with survival in both univariate and multivariate analyses with p-values less than 0.05.
In summary, the authors generated a prognostic index model to predict the chance of benefitting from Ra-223 therapy and identified several pre-treatment and treatment-related factors associated with survival in this context. External validation is required prior to potential incorporation of this model into clinical decision making.
Reference:
Presented by: Esmail Al-Ezzi, MD, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada
Written by: Alok Tewari, MD, Ph.D., Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020


