(UroToday.com) As a portion of the European Society for Medical Oncology (ESMO) Annual Congress, an Educational Session focused on improving outcomes in bladder cancer was held. In this context, Dr. Cathomas discussed how to select the optimal first line systemic therapy in advanced urothelial disease.
Dr. Cathomas began highlighting both patient- and disease-factors that may be important in choosing treatment. In terms of patient characteristics, we must consider whether a patient is platinum-eligible; if platinum eligible, whether they are cisplatin-eligible; their age; and potentially their gender. In terms of disease characteristics, we can consider the tumor PD-L1 status, other predictive biomarkers, the effect of prior neoadjuvant or adjuvant therapy, and variant histologies.
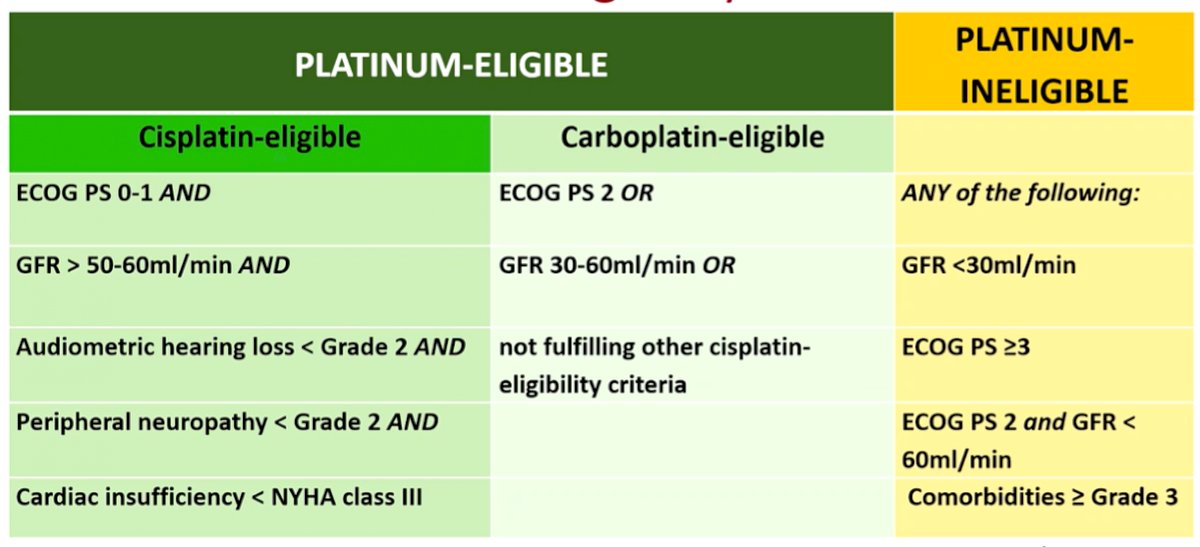
He then emphasized that the single most relevant factor for treatment selection is whether a patient is platinum, and particularly cisplatin, eligible. He highlighted a number of criteria, based on work by Dr. Galsky, which define a patient at eligible for cisplatin. Up to 50% of patients with metastatic urothelial carcinoma (mUC) are cisplatin-ineligible. However, the majority of these will be eligible for carboplatin. Approximately 10-15% of patients with mUC will not be eligible for carboplatin and therefore must be considered for other treatment approaches.
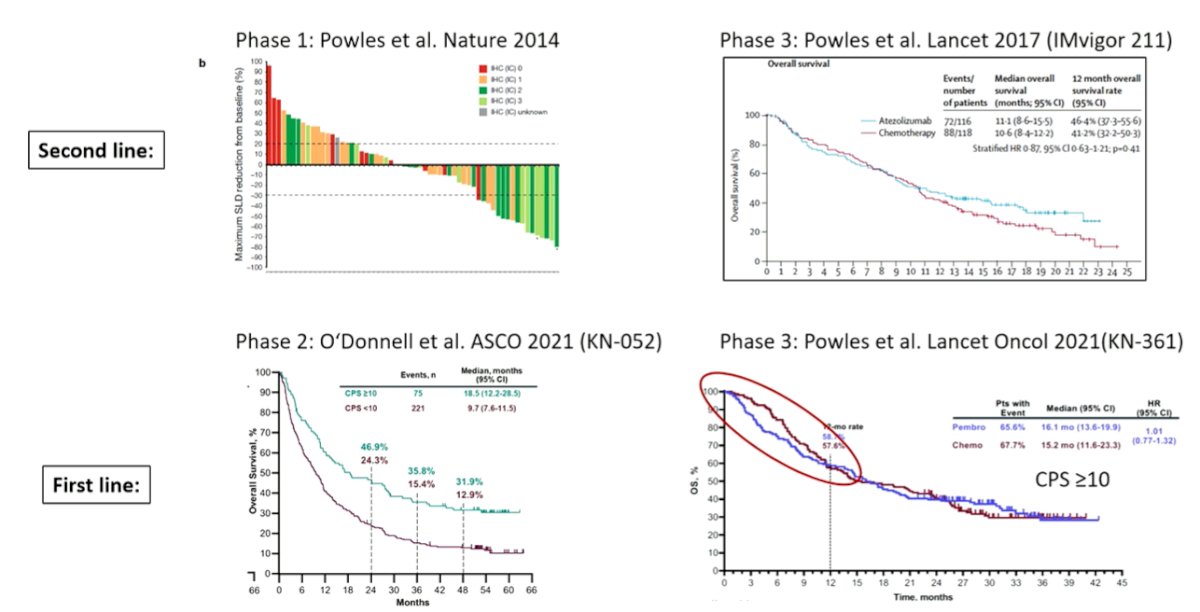
Based on two retrospective analyses, he demonstrated that the median overall survival for patients who are cisplatin eligible but did not receive cisplatin is meaningfully shorter (difference ~ 6 to 7.5 months) than those who actually received cisplatin. However, this focused on the first-line setting and he then emphasized, based on data from DANUBE, KEYNOTE-361, and IMVigor130, that in the second and third lines of therapy, carboplatin may have more activity than expected. Thus, while cisplatin is preferred, the platinum choice is likely less important for patients receiving treatment beyond first-line therapy.
Dr. Cathomas then considered the question of patient gender. While there is an extensive body of literature examining disparities in care for women with bladder cancer including a longer time to diagnosis, higher age and stage at diagnosis, there is no data to suggest that patient gender should affect treatment choice. In particular, chemotherapy trials have not demonstrated a differential effect of gender on outcomes. However, a numerically lower response to immunotherapy has been demonstrated.
In terms of age, he emphasized that urothelial carcinoma is predominantly a disease of the elderly. As expected, trials skew somewhat to a younger age but are still enriched with the majority of patients being age 65 years and older. Among patients who fulfil cisplatin-eligibility, there is no evidence that older age is associated with worse outcomes therefore, independently, age should not be considered to influence treatment selection.
Dr. Cathomas then transitioned to discussing disease factors, focusing first on PD-L1 which he described as the most important biomarker currently in this disease. Initial work raised the hope that PD-L1 expression would be predictive for response to atezolizumab. However, this was not borne out in phase III trials.
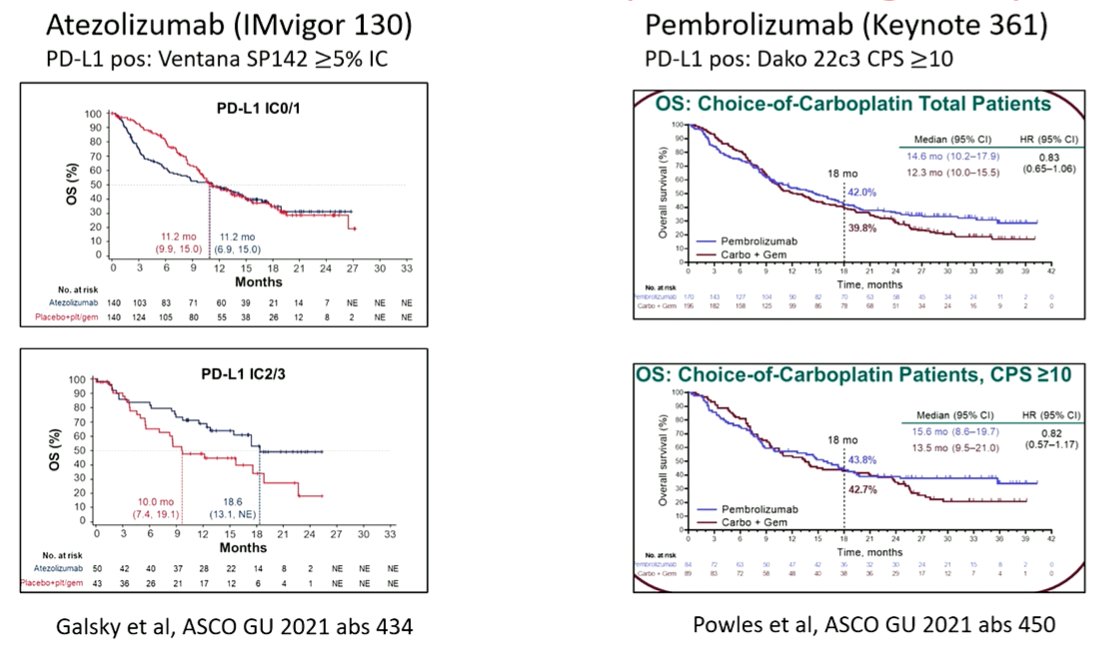
He then considered if, instead, PD-L1 expression may be more useful when examining treatment options among patients who are cisplatin-ineligible. There was hope that PD-L1 would predict for a benefit of immunotherapy compared to a carboplatin based chemotherapy regime. However, while this was assessed in IMvigor130 and KEYNOTE-361, the results are conflicting. In IMvigor130, treatment with atezolizumab was superior to carboplatin-gemcitabine in the subgroup of PD-L1 positive patients with not those who were PD-L1 negative. However, no such evidence of effect modification was seen in KEYNOTE-361. He emphasized that these analyses should be interpreted with caution given small sample sizes.
While these checkpoint based approaches are approved for cisplatin-ineligible patients, he emphasized that a carboplatin-gemcitabine approach remains his preferred treatment regime for most patients.
He then moved on to discuss integration of immunotherapy in the first line. As assessed in three trials (KEYNOTE-361, DANUBE, and IMVigor130), there is no evidence of overall survival benefit for the concurrent use of immunotherapy and chemotherapy or doublet immunotherapy compared to standard chemotherapy. However, based on the JAVELIN Bladder 100 trial, for those patients who have a partial response or stable disease following initial induction chemotherapy, switch maintenance to avelumab is associated with an overall survival benefit.
Dr. Cathomas then presented a potential practical algorithm for the integration of neoadjuvant and adjuvant treatment regimes into treatment selection for patients with mUC. As highlighted in the flow diagram below, whether a patient has early (<12 month) or late (>12 month) relapse may influence treatment choice. In general, a switch treatment approach is a reasonable approach. However, platinum re-challenge is also reasonable for those with late relapse.
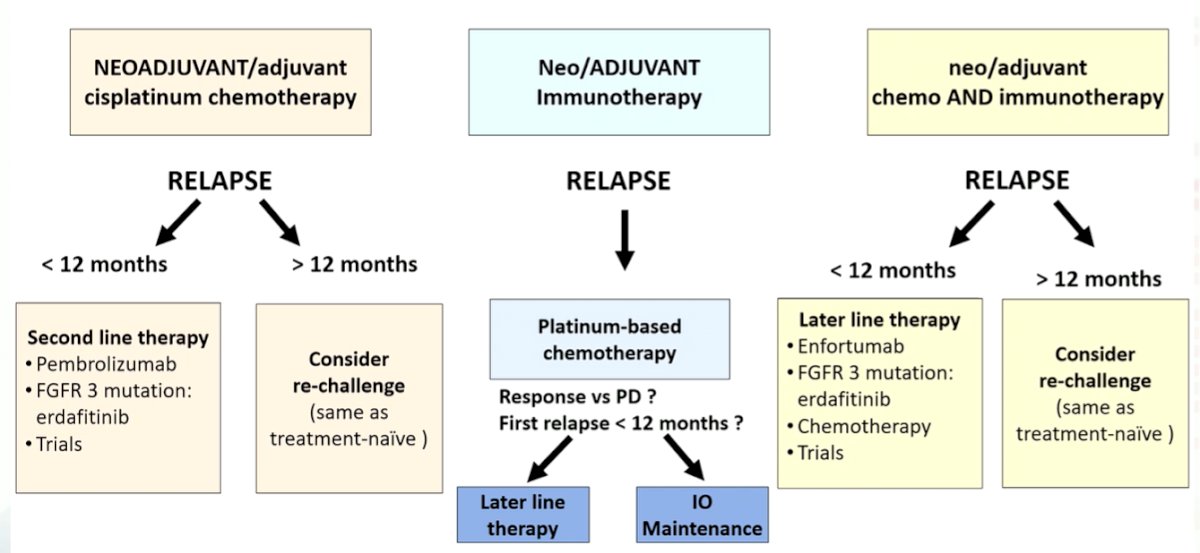
He further emphasized that this algorithm is likely to change as new treatment options, including targeted therapies, become used earlier in the disease process.
Finally, he considered the role of variant histology in treatment decision making. While these variants are relatively common, comprising up to 33% of patients, there is very little evidence to guide how they should affect treatment choice. In general, mixed variants are treated the same are pure urothelial carcinoma, with some notable exceptions for those with small cell neuroendocrine carcinoma, pure adenocarcinoma, and pure squamous cell carcinoma.
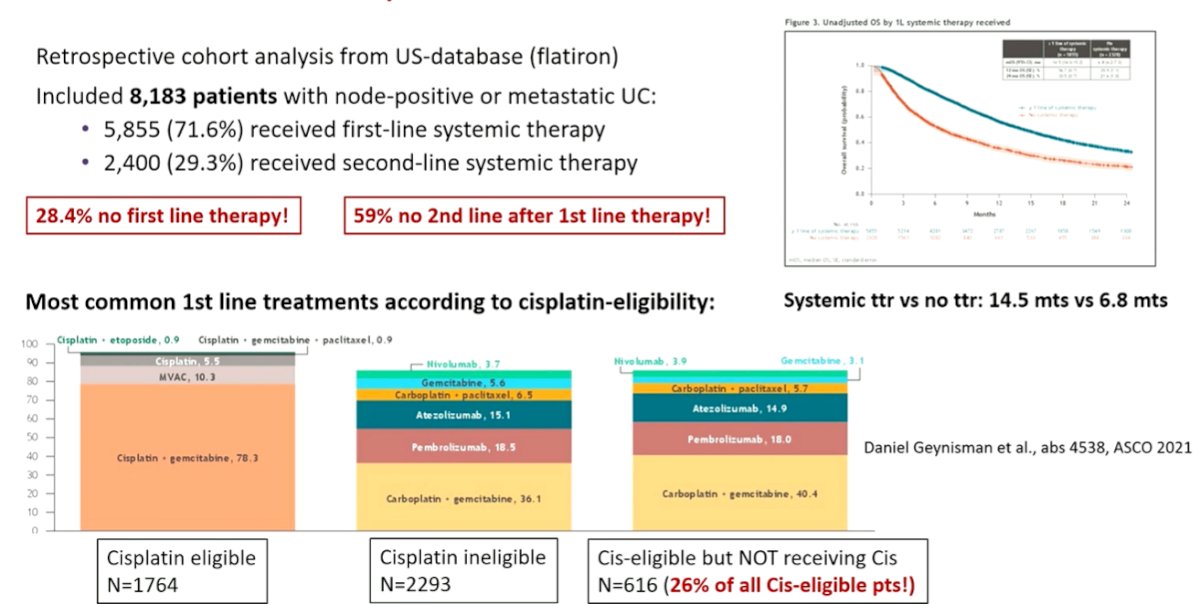
He then emphasized that while there has been significant developments in treatment options within clinical trials, there has been a significant issue with translating this to routine clinical care. In a recent study of the US-based Flatiron database, 28% of patients with node-positive or metastatic urothelial carcinoma did not receive first-line systemic therapy and 59% of those who receive first-line therapy did not receive second-line therapy. This was associated with significant differences in survival (difference in median nearly 8 months). Further, among those who were cisplatin-eligible, many did not receive chemotherapy.
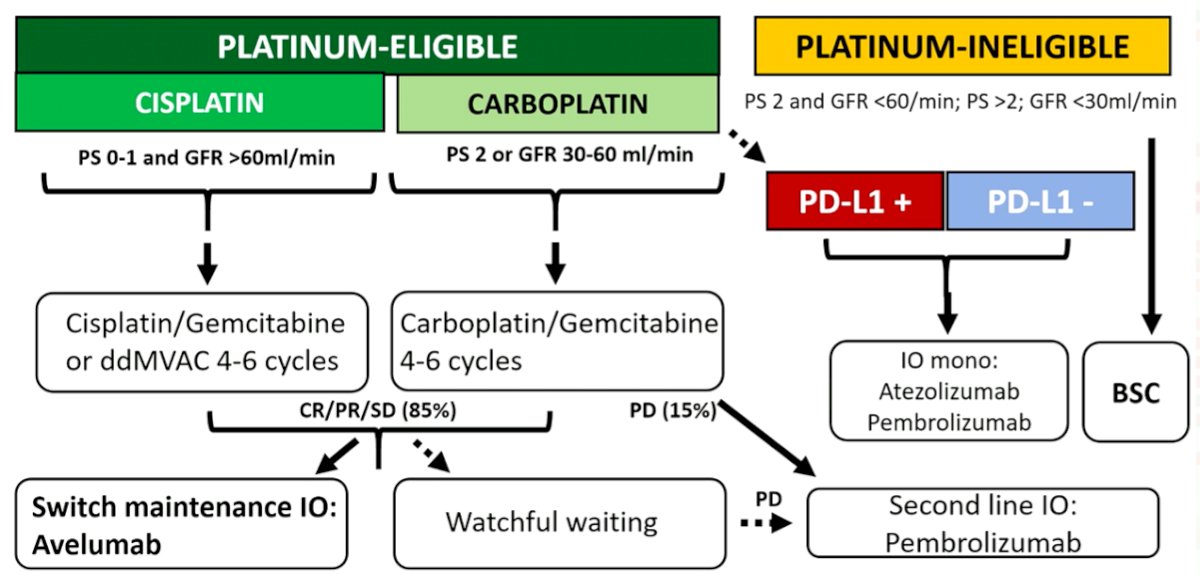
He concluded by proceeding through a proposed algorithm for first-line treatment of mUC, emphasizing the importance of platinum-eligibility. For those who are platinum-ineligible, there is low level evidence to guide care. However, immunotherapy monotherapy may be considered regardless of PD-L1 status in the United States.
Presented by: Richard Cathomas, MD, PhD, Department of Oncology, Kantonsspital, Graubünden, Switzerland


