(UroToday.com) In the on-demand poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Jonathan Rosenberg presented a subgroup analysis of the EV-301 trial. This phase III trial (NCT03474107) demonstrated that treatment with enfortumab vedotin (EV) showed superior overall survival (OS) compared with standard chemotherapy (SC) in patients with previously treated locally advanced or metastatic urothelial carcinoma (la/mUC). This subgroup analysis reports on the efficacy and safety of EV for those with poor prognostic factors.
To summarize, this open-label, phase III trial, included patients with la/mUC who were previously treated with platinum-based chemotherapy and a PD-1/L1 inhibitor. These patients were randomized 1:1 to EV or the investigator’s choice of SC (docetaxel, paclitaxel, vinflunine).
In this presentation, Dr. Rosenberg provides prespecified subgroup analyses for the primary endpoint of OS and secondary endpoints of investigator-assessed progression-free survival (PFS) and overall response rate (ORR) per RECIST v1.1. Kaplan-Meier analyses compared OS and PFS between treatments for selected hard-to-treat subgroups: age ≥65 years, presence of liver metastasis, primary upper tract disease, and checkpoint inhibitor (CPI) nonresponse. ORR and safety were also evaluated within subgroups.
In the EV-301 trial, as of July 15, 2020, 301 patients were randomized to EV and 307 were randomized to SC. The median follow-up was 11.1 months. OS benefit of EV was retained across most hard-to-treat subgroups with longer median OS in pts on EV vs SC.
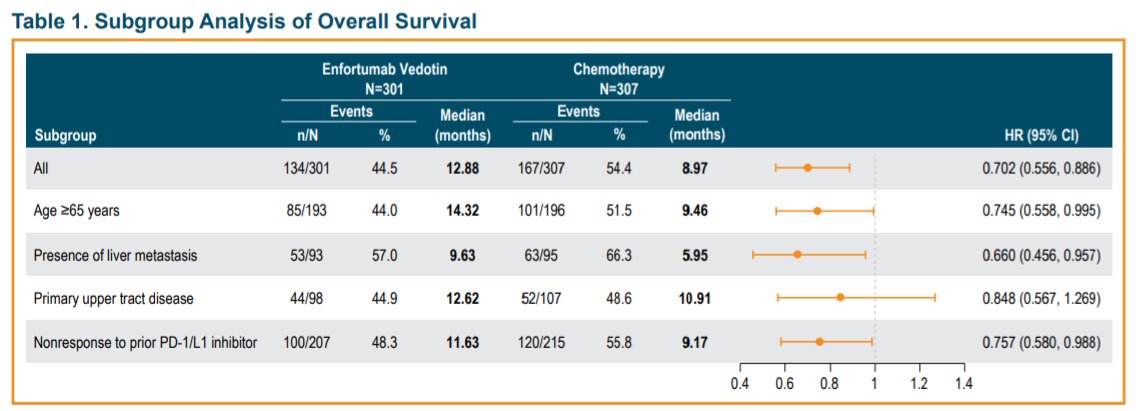
Additionally, PFS and ORR benefit was also observed across most subgroups for EV vs SC. However, overall rates of treatment-emergent and -related adverse events were comparable between treatments across subgroups.
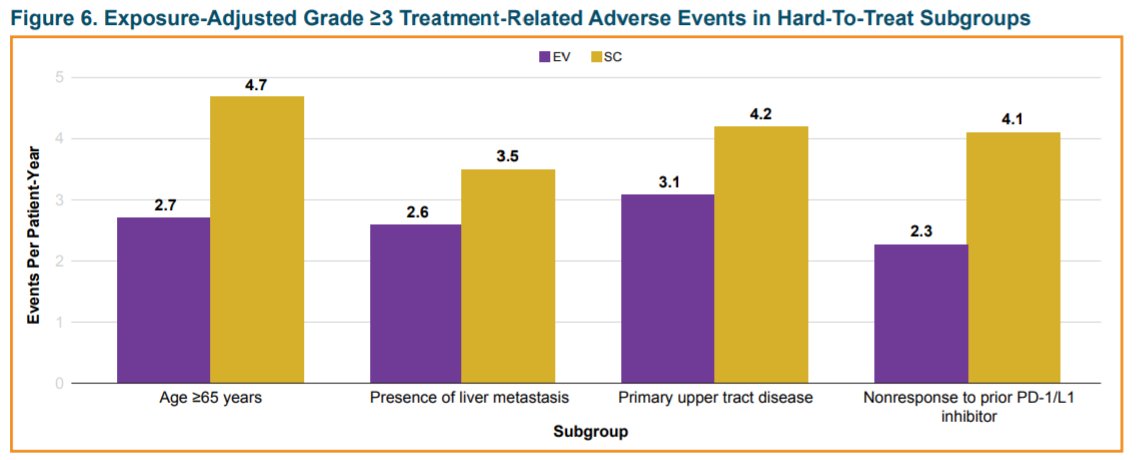
Thus, he concluded that subgroups of patients with la/mUC who may be hard-to-treat had consistently longer OS and PFS and higher ORR with EV vs SC.
Presented by: Jonathan E. Rosenberg, MD, Chief of the Genitourinary Medical Oncology Service, Division of Solid Tumor Oncology; and the Enno W. Ercklentz Chair at Memorial Sloan Kettering Cancer Center, New York City, New York


