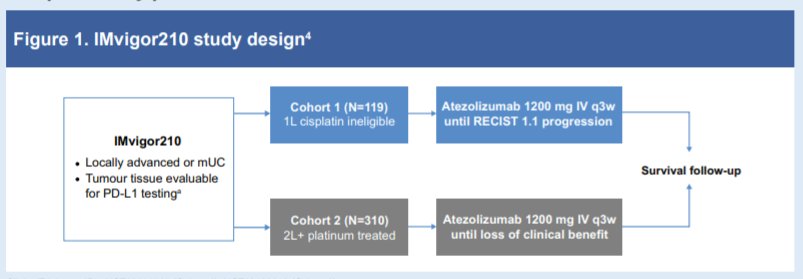
(UroToday.com) In the on-demand poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Rosenberg reported long-term follow-up of the IMvigor210 Cohort 1. While cisplatin-based chemotherapy is the standard of care for first-line (1L) metastatic urothelial carcinoma (mUC), many patients are ineligible for treatment with cisplatin. Thus, Cohort 1 of the single-arm phase II IMvigor210 study evaluated the efficacy and safety of first-line atezolizumab (anti–PD-L1) in cisplatin-ineligible patients with mUC. These data formed the basis of the ongoing randomised phase III IMvigor130 study.
To summarize the previously published IMVigor210 Cohort 1, cisplatin-ineligible patients with untreated mUC received atezolizumab monotherapy 1200 mg IV q3w until disease progression by RECIST 1.1 or toxicity. The authors assessed the primary endpoint of confirmed objective response rate (ORR; RECIST 1.1) per independent review facility (IRF). Secondary endpoints included duration of response (DOR) and overall survival (OS). In this analysis, efficacy by PD-L1 status on tumour-infiltrating immune cells (VENTANA SP142 IHC assay) was descriptively evaluated.
The authors examined 119 evaluable patients who had a minimum follow-up of 5.8 years as of the data cutoff of 31 January 2021. The median DOR was 59.1 months (approximately 5 years) in the overall population and 53.5 months in pts with PD-L1 IC0/1 tumours (IC2/3 median DOR not yet reached).
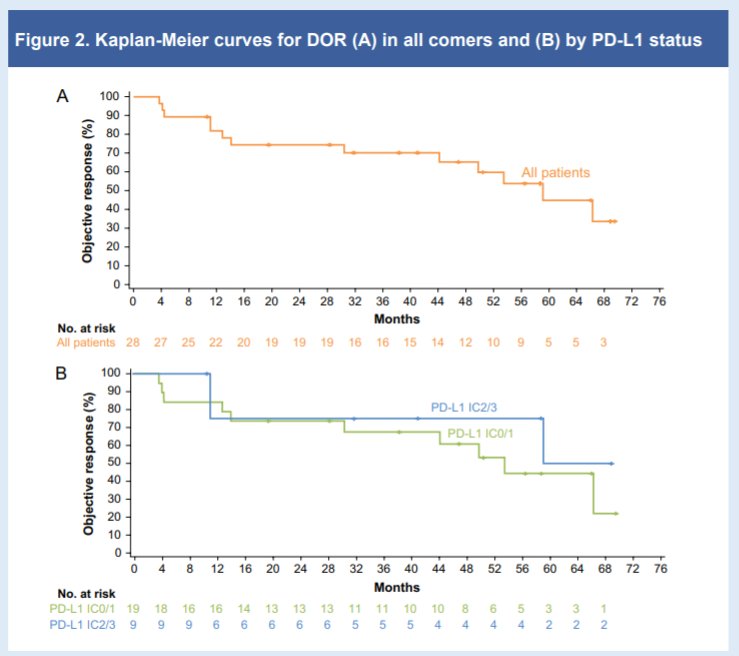
The authors, therefore, conclude that prolonged (>5 years) follow-up of the IMvigor210 Cohort 1 in cisplatin-ineligible mUC demonstrates that first-line treatment with atezolizumab monotherapy is associated with durable responses and long-term survival.


