(UroToday.com) The European Society of Medical Oncology (ESMO) 2021 meeting’s proffered paper session for non-prostate genitourinary tumors included a discussant presentation by Dr. Srikala Sridhar of the phase 2 NORSE trial assessing erdafitinib or erdafitinib plus cetrelimab for patients with metastatic or locally advanced urothelial carcinoma and fibroblast growth factor receptor (FGFR) alterations. Dr. Sridhar started by highlighting the treatment landscape of metastatic urothelial carcinoma as it stands in 2021, noting that we now have first through fourth-line therapies, stratified by platinum eligible versus ineligible:

The FGFR pathway in metastatic urothelial carcinoma has emerged as an actionable pathway for targeted therapy. Mechanistically, there are five receptor tyrosine kinases that bind FGF, which activates downstream signaling, leading to cell proliferation, differentiation, migration, and survival. Ultimately, dysregulated signaling can lead to oncogenesis. In metastatic urothelial carcinoma, 15-20% of tumors have FGFR alterations, common in luminal papillary subtypes, upper tract tumors, and associated with less T-cell infiltration. A summary of selected FGFR inhibitors in metastatic urothelial carcinoma are as follows, including erdafitinib, rogaratinib, infigratinib, and pemigatinib:

There is conflicting data existing on FGFR alterations and response to immunotherapy, with prior studies suggesting a lower response to immunotherapy. Previous work notes no difference in objective response rate in FGFR wild type versus mutation, and FGFR knockdown upregulates IFN gamma response genes. Thus, combining FGFR and PD-1/L1 inhibitors may be a logical therapeutic approach, with increased efficacy, reverse resistance, address heterogeneity, and target the microenvironment.
Dr. Sridhar notes that there are several open questions with regards to combination approaches:
- Is there good preclinical rationale?
- What is the ideal patient population in which to test the combination?
- Is the combination tolerable?
- Can both drugs be dosed appropriately?
- What are the appropriate endpoints?
- Is efficacy better, same or possibly worse?
- If better, is there an additive or synergistic benefit?
- Is there a clinically significant improvement?
- Is combination better than sequential?
- Are there biomarkers of efficacy and toxicity?
In addition to the NORSE trial, there are several first-line, cisplatin-ineligible trials of FGFR inhibitors and immunotherapy:
- NORSE (FGFR3 mutation/fusions): erdafitinib + cetrelimab versus erdafitinib
- FORT-2 (FGFR1/3 mRNA overexpression): rogaratinib + atezolizumab versus atezolizumab + placebo
- FIGHT-205 (FGFR mutation/fusions): pemigatinib + pembrolizumab versus pemigatinib versus standard of care
Dr. Sridhar notes that biomarker-guided trials, such as the NORSE trial, are challenging given that many patients need to be screened, there may be issues with tissue availability, quality, quantity, and heterogeneity, and there may be delays in treatment. So, can ctDNA be an option since it is convenient, rapid, and non-invasive? The BLC2001 trial testing erdafitinib in the second-line setting demonstrated a 63% concordance rate between ctDNA and tissue-based testing,1 and the BISCAY trial assessing AZD4547 + durvalumab in the second-line setting showed that sequential ctDNA analysis demonstrated changes in FGFR alterations that correlated with clinical outcomes.2 Thus, it seems reasonable to consider incorporating parallel ctDNA analysis into prospective trials, both at screening and for monitoring response.
Dr. Sridhar then highlighted several key points from the NORSE trial regarding baseline characteristics. First, it is encouraging to see inclusion of older adults, since often these individuals are excluded from clinical trials. In both treatment arms of the NORSE trial, there were patients in their early 90s. Second, a significant proportion of patients in both arms (54% erdafitinib arm; 67% erdafitinib + cetrelimab arm) had an unknown PD-L1 status secondary to a lack of tissue. Third, there was a high incidence of FGFR alterations (88% mutations, 12% fusions for erdafitinib arm; 67% mutations, 26% fusions, 4% mutations for erdafitinib + cetrelimab arm), compared to 49% FGFR3 mutations and 16% FGFR2/3 fusions in the BLC2001 trial. With regards to toxicity, there was a similar safety profile in each arm, consistent with the phase 1 trial and the FORT-2 study. Hyperphosphatemia may be a biomarker of response with a single agent, and eye toxicity is quite common and requires close monitoring. Discontinuation rates were higher in the combination arm, but efficacy appears to be maintained.
Dr. Sridhar emphasized that the objective response rate of 68% for erdafitinib + cetrelimab is impressive and that the combination therapy appears to be better than erdafitinib alone, but cautions that these are small numbers with as of yet a progression-free survival or overall survival benefit. Compared to the BISCAY trial [1], response rates ranged from 9-36% across the study arms, which did not meet efficacy criteria for further development. Additionally, combination had a lower response rate than the single agent in the platinum-refractory setting. Dr. Sridhar notes that it is curious that two studies with similar designs and similar drugs showed one positive trial and the other negative. As such, perhaps the setting matters (cisplatin-unfit versus refractory), and the type of FGFR inhibitor makes a difference. Biomarkers will likely assist in identifying patients, with the NORSE trial noting responses in both FGFR mutations and fusions, as well as in low PD-L1 status patients.
Compared to other first-line, cisplatin-ineligible trials the objective response rate is quite impressive, summarized in the following efficacy table:
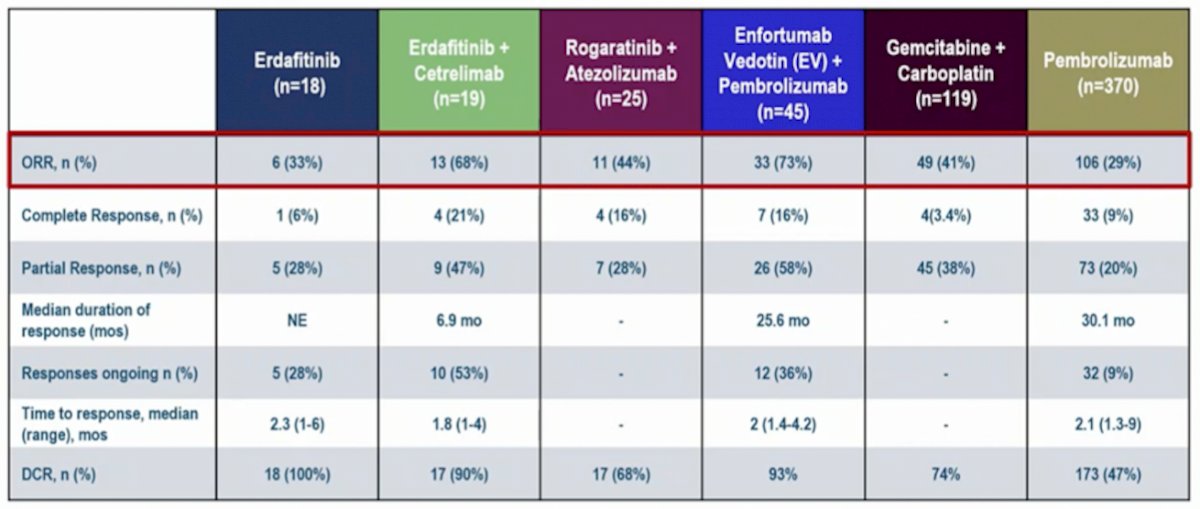
Dr. Sridhar summarized the NORSE study with the following points:
- Does this study change practice? Currently, no it does not in her opinion
- Strengths: this builds on strong preclinical work, has a randomized design, continues to accrue, early results for the combination are encouraging, and responses were seen in patients regardless of FGFR mutation, fusion, or PD-L1 status
- Caveats: the study has small numbers, short follow-up, and Dr. Sridhar is unsure about objective response rate as an endpoint. Furthermore, the comparator is not standard of care. The prior BISCAY study in the platinum-refractory setting was negative, but why is this study positive?
- Future studies: Perhaps we should be randomizing against immunotherapy or chemotherapy or enfortumab vedotin + pembrolizumab, which could be the new first-line standard in this setting, although we are unsure when
- Unanswered questions:
- What is best, an additive or synergistic approach?
- What is the optimal biomarker strategy?
- How do FGFR alterations impact response to chemotherapy?
- How do FGFR alterations impact response to antibody-drug conjugates?
- What are mechanisms of resistance?
- How do we best sequence with other agents?
- Will evaluation in earlier stages of the disease, for example, the neoadjuvant setting lead to cures, and allow for pre- and post-treatment correlative studies?
Finally, Dr. Sridhar concluded her presentation with the following take-home messages:
- Novel therapies continue to be explored across the spectrum of urothelial cancers
- Single-agent and combination based strategies hold promise for improved outcomes in terms of both efficacy and tolerability, but trial design is very important
- Biomarkers are urgently needed to help us not only with patient selection but also to understand response, resistance, and toxicities
- As more options become available, we need trials to help us know how best to sequence agents to optimize outcomes
Presented by: Srikala Sridhar, MD, Princess Margaret Cancer Center, Toronto, Ontario
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References:
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2019 Jul 25;381(4):338-348.
- Powles T, Carroll D, Chowdhury S, et al. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat Med. 2021 May;27(5):793-801.


