(UroToday.com) In this presentation, Dr. Thomas Powles presented results from the Phase 2 NORSE study of erdafitinib or erdafitinib plus cetrelimab in patients with metastatic or locally advanced urothelial carcinoma (UC) and fibroblast growth factor receptor (FGFR) alterations. These preliminary results discussed by Dr. Thomas Powles focused on overall response rate (ORR) and safety data.
The standard of care for newly diagnosed metastatic UC is cisplatin-based chemotherapy, however, more than 50% of patients are ineligible for cisplatin treatment. There is a significant unmet need for new treatments for patients with metastatic UC who are cisplatin-ineligible. Treatment options for patients with newly diagnosed metastatic UC who are ineligible for cisplatin include alternative chemotherapy regimens of anti-PD-(L)1 monotherapy for patients with PD-L1-positive tumors. Carboplatin-based therapy is thought to be inferior to cisplatin-based therapy. PD-L1 positivity rates range from 30-60%, highlighting the fact that many patients may not be eligible for first-line immunotherapy.
Approximately 15-20% of patients with metastatic UC have an alteration in FGFR2. erdafitinib is FDA approved for adults with metastatic UC with a susceptible FGFR2/3 alteration who progressed after one or more prior lines of platinum-based chemotherapy. It is hypothesized the treatment with erdafitinib may release neoantigen and prime the tumor microenvironment for response to immunotherapy. This provides the rationale for combing erdafitinib with a checkpoint inhibitor. cetrelimab is a PD-1 inhibitor, not yet DFA approved, that has been administered to 370 patients across several clinical trials with safety data consistent with other PD-1inhibitors.
The NORSE study includes patients with metastatic UC who have not yet received any systemic therapy, are ineligible for cisplatin, and who harbor and FGFR alteration (mutation or fusion). Patients are randomized to receive erdafitinib or erdafitinib plus cetrelimab. The co-primary endpoints are overall response rate (ORR) and safety.
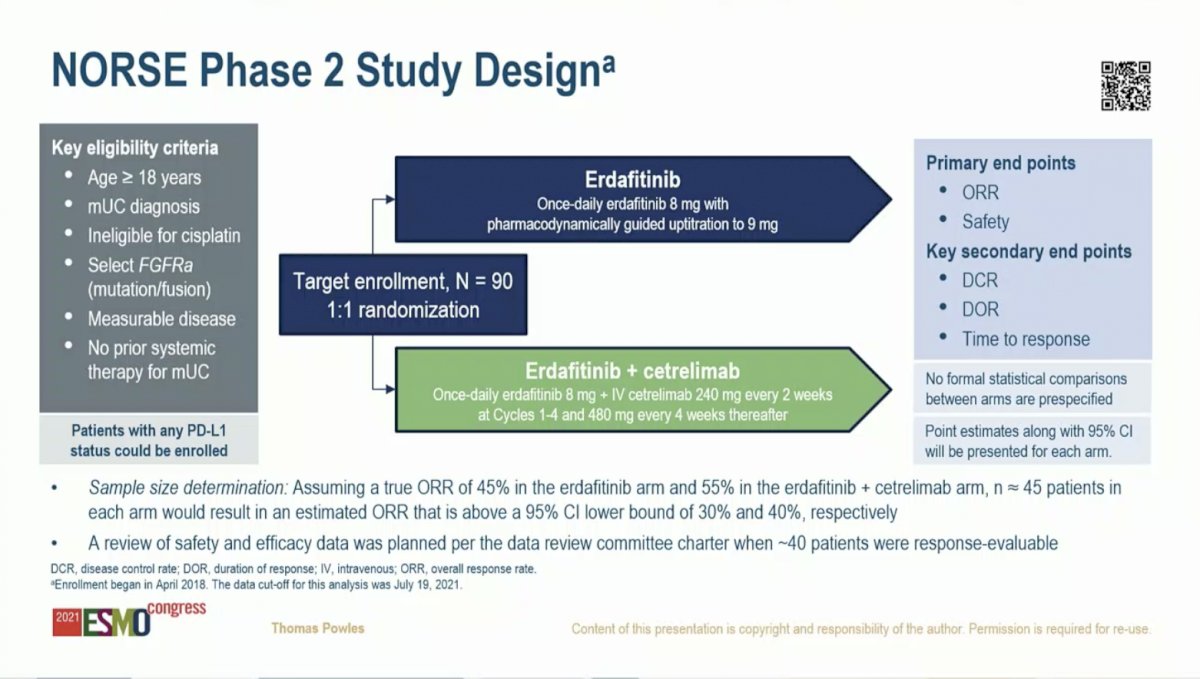
To date, 53 patients have been randomized with 48 evaluable for the safety analysis and 37 evaluable for the efficacy analysis. The two arms were largely balanced for demographic and baseline characteristics. However, patients on the combination arm were younger (69 versus 75) and had a higher rate of ECOG PS 2 (33% vs 23%). The rate of PD-L1 positivity was similar on the two arms. Notably, patients on the erdafitinib arm had a higher rate of mutations (88%) compared to the combination arm (67%). This is potentially significant as published data suggest that patients harboring FGFR mutations have a higher response rate to erdafitinib than those with FGFR fusions.1

The preliminary efficacy analysis favored erdafitinib plus cetrelimab over erdafitinib alone. The ORR was 68% for the combination compared to 33% for erdafitinib. Likewise, the complete response rate was 21% versus 6% in favor of the combination.

Patients in both treatment arms had a durable reduction in the sum of target lesion diameter over time. The median of the maximum reduction in the sum of target lesion diameters was 28% in the erdafitinib arm and 51% in the erdafitinib plus cetrelimab arm. Despite prior data suggesting higher response rates in patients with FGFR mutations as compared to fusions, responses were seen on the combination arm for patients with either type of alteration. Responses were seen on both arms for PD-L1 low patients. Too few PD-L1 positive patients had response data for analysis at this time.

Safety analysis revealed that the treatment-emergent adverse event (AE) profile was similar for erdafitinib plus cetrelimab compared to that of erdafitinib alone. Hyperphosphatemia was the most common AE on both arms, followed by nail, skin, and eye toxicity. The most frequent Grade 3/4 AEs (all present in 12.5% of patients) were anemia and general physical health deterioration on the combination arm and stomatitis and increased lipase on the erdafitinib arm. There was one death on the combination arm from respiratory failure, which was thought to be related to cetrelimab. Treatment discontinuation rates of either erdafitinib or cetrelimab on the combination arm were higher (29%) than discontinuation of erdafitinib (8%) on the monotherapy arm.


Dr. Powles concluded that these initial data on the combination of erdafitinib plus cetrelimab in patients with newly diagnosed metastatic UC who are ineligible for cisplatin-based therapy and harbor an FGFR alteration suggest that the combination may improve the ORR and depth of response compared to erdafitinib alone. Clinical activity was observed in both patients with PD-L1 high and low status, however, the PD-L1 status was not known for all patients at the time of analysis. Overall, safety with erdafitinib plus cetrelimab was generally consistent with erdafitinib alone and aligned with the known safety profile of approved anti-PD-1 therapies, however, drug discontinuation occurred more frequently on the combination arm than the monotherapy arm. Enrollment in this study is ongoing. In sum, these data suggest potential positive interaction between erdafitinib and cetrelimab, possibly mediated by priming the immune environment via neoantigen release in patients with an FGFR alteration to increase response to immune checkpoint blockade.
Presented by: Thomas B. Powles, MBBS, MRCP, MD Professor of Genitourinary Oncology, Director, Barts Cancer Centre, Lead for Solid Tumour Research
Written by: Jacob Berchuck, MD, Genitourinary Medical Oncologist, Dana-Farber Cancer Institute (Twitter: @jberchuck) during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References:


