(UroToday.com) In this presentation, Dr. Srikala Sridhar discussed results from the Phase 2 NORSE study of erdafitinib or erdafitinib plus cetrelimab in patients with metastatic or locally advanced urothelial carcinoma (UC) and fibroblast growth factor receptor (FGFR) alterations. This study addresses a significant unmet clinical need for better first-line therapy options for patients with metastatic UC who are not eligible for cisplatin-based therapy.
Alterations in FGFR genes are present in approximately 15-20% of patients with metastatic UC. These tumors are enriched in patients with luminal papillary subtype and upper tract tumors. There are several FGFR inhibitors in various stages of clinical development, but erdafitinib is the only one FDA approved for patients with UC. Specifically, erdafitinib is approved for patients with metastatic UC harboring FGFR2/3 alterations who have progressed on prior platinum chemotherapy.1

Conflicting data exist on how FGFR alterations impact response to immunotherapy. Prior studies, IMVigor 210 and CheckMate 275 found no difference in the overall response rate to atezolizumab or nivolumab, respectively, based on FGFR alteration status. Yet, pre-clinical data suggest that combining FGFR and PD-(L)1 inhibitors may be an effective therapeutic approach. Dr. Sridhar noted that the NORSE study is not the only clinical trial investigating this approach as first-line therapy in patients with cisplatin-ineligible metastatic UC.

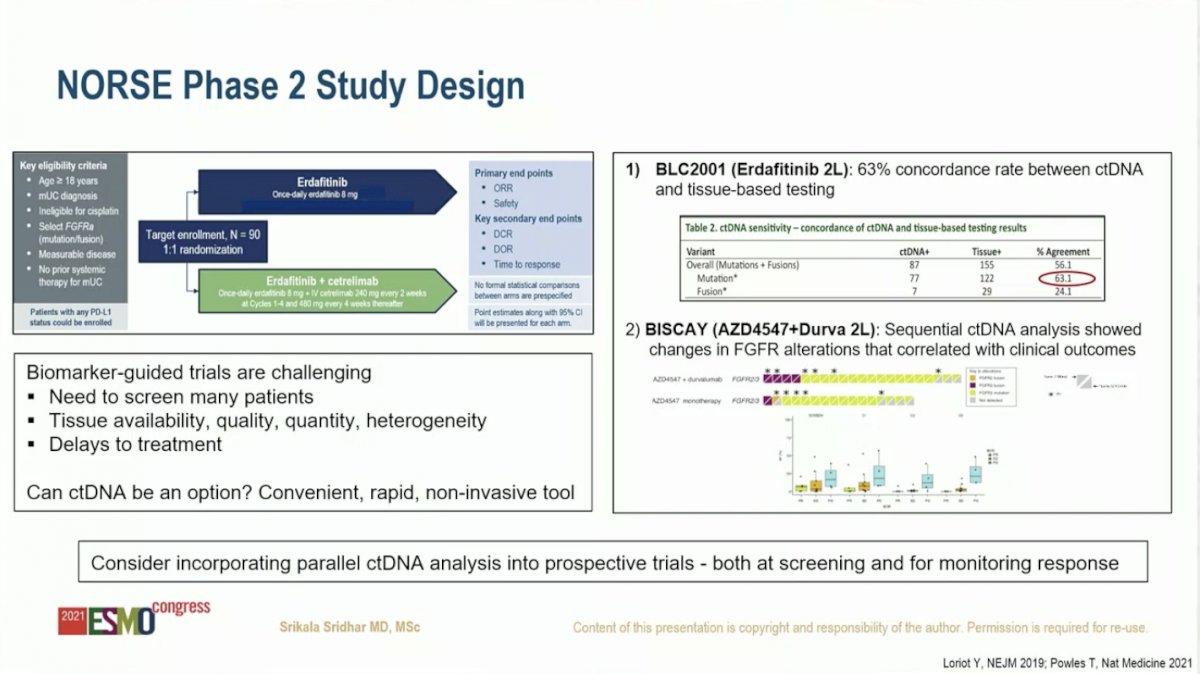
In discussing the NORSE study design, Dr. Sridhar highlighted the challenges of conducting a biomarker-guided trial, including the need to screen many patients, lack of tissue availability of sufficient quantity and quality, and delays in treatment. She posited that circulating tumor DNA (ctDNA) analysis could be a convenient, rapid, non-invasive tool to overcome these issues. In the BLC2001 study, which led to FDA approval of erdafitinib in UC, there was 63% concordance between ctDNA and tumor tissue for identifying FGFR alterations. Further, sequential ctDNA monitoring was an effective tool for predicting clinical outcomes. As such, Dr. Sridhar recommended that ctDNA be incorporated into prospective trials both for screening patients as well as monitoring response to therapy.
Dr. Sridhar next focused on the first of two primary endpoints – safety. She noted an overall similar toxicity profile in each arm. Hyperphosphatemia was the most common adverse event (AE) on both arms. She highlighted data from prior studies suggesting that this on-target side effect may be associated with response for single agent erdafitinib. Whether this will be seen for erdafitinib plus cetrelimab is not known. She also highlighted eye toxicity as a common side effect and urged multidisciplinary care to monitor for this unique AE. Lastly, Dr. Sridhar mentioned that although discontinuation rates were higher in the combination arm, this did not appear to compromise efficacy.

Next, Dr. Sridhar reviewed the second primary endpoint, overall response rate (ORR). Acknowledging the limitation of the small sample size, the combination of erdafitinib plus cetrelimab resulted in higher ORR (68%) compared to erdafitinib alone (33%). Likewise, the complete response rate favored the combination (21%) versus monotherapy (6%). The high response rates raise the question of whether the combination increases the number of patients who may benefit compared to each drug alone. Interestingly, these positive results are in contrast to the BISCAY study, which found a lower response rate for an FGFR inhibitor plus PD-L1 inhibitor. Notably, this study enrolled patients in the platinum-refractory setting whereas patients treated on the NORSE study were systemic therapy naïve. This raises questions about factors that may contribute to the discrepant results between the NORSE and BISCAY studies, including patient selection (cisplatin-ineligible versus platinum refractory), type of FGFR inhibitor, and PD-1 versus PD-L1.

Finally, Dr. Sridhar compared the ORR results from the NORSE study with other clinical trials in the first-line setting for cisplatin-ineligible patients with metastatic UC. The ORR of 68% for erdafitinib plus cetrelimab is amongst the highest reported in the setting, nearing the 73% for patients treated with enfortumab vedotdrin plus pembrolizumab on the EV-103 study. The complete response rate of 21% for erdafitinib plus cetrelimab is the highest reported in this setting to date.

Dr. Sridhar concluded that, while not practice-changing, this study builds on strong preclinical work to demonstrate a novel combination with encouraging disease activity in the first-line setting for cisplatin-ineligible patients. However, given the small sample size and lack of progression-free and overall survival data, further, follow-up is needed. As additional data for this promising combination emerges, Dr. Sridhar is interested to see whether it is additive vs synergistic, optimal biomarker strategies, mechanisms of resistance, and how to best sequence with other agents.
Presented by: Srikala Sridhar, MD, MSc, FRCPC, Associate Professor at University of Toronto, Medical Oncologist at Princess Margaret Cancer Centre
Written by: Jacob Berchuck, MD, Genitourinary Medical Oncologist, Dana-Farber Cancer Institute (Twitter: @jberchuck) during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References:


