(UroToday.com) The European Society of Medical Oncology (ESMO) 2021 virtual annual meeting’s non-prostate cancer session included a presentation by Dr. Brian Rini discussing a new 3-arm phase 3 trial concept and design, which will randomize patients with advanced clear cell RCC in the first-line setting to either pembrolizumab + belzutifan + lenvatinib versus MK-1308A + lenvatinib versus pembrolizumab + lenvatinib. Despite advances in treatment, most patients with advanced clear cell RCC will eventually experience disease progression on treatment. Combination therapy with the PD-1 inhibitor pembrolizumab and the VEGF inhibitor lenvatinib showed activity in patients with advanced ccRCC in the phase III KEYNOTE-581/CLEAR trial.1 The HIF-2α inhibitor belzutifan (MK-6482) and MK-1308A, a coformulation of pembrolizumab and the CTLA-4 inhibitor quavonlimab, have each shown antitumor activity in phase I/II trials. HIF-2α or CTLA-4 inhibition with PD-1 and VEGF inhibition backbone combination may provide additional benefit as first-line treatment in clear cell RCC.
This open-label, phase III study (NCT04736706) is currently enrolling patients and will compare two new combination therapies with pembrolizumab + lenvatinib (control): pembrolizumab + belzutifan + lenvatinib (HIF arm) or MK-1308A + lenvatinib (CTLA arm). Eligible patients are adults with metastatic clear cell RCC, measurable disease per RECIST v1.1, KPS ≥ 70%, and no prior systemic therapy for advanced clear cell RCC. Patients will be stratified by IMDC score (0 versus 1/2 versus 3-6) and region (North America versus Western Europe versus rest of the world). The study design for this trial is as follows:
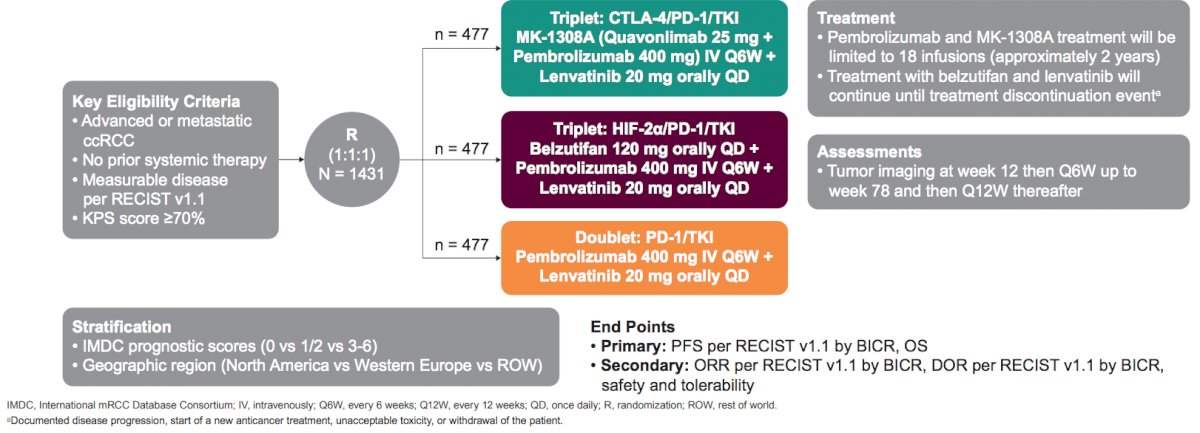
The study will enroll 1,431 patients, randomly assigned 1:1:1 to the HIF arm (belzutifan 120 mg + lenvatinib 20 mg oral QD + pembrolizumab 400 mg IV Q6W), CTLA-4 arm (MK-1308A [quavonlimab 25 mg + pembrolizumab 400 mg] IV Q6W and lenvatinib 20 mg oral QD), or the control arm (pembrolizumab 400 mg IV Q6W + lenvatinib 20 mg oral QD). Treatment will be given until documented disease progression, withdrawal of consent, or other discontinuation event; pembrolizumab and MK-1308A will be limited to 18 infusions (2 years). Response will be evaluated per RECIST v1.1 by blinded-independent central review with CT/MRI imaging at week 12 from randomization through week 78, and Q12W thereafter. Adverse events and serious adverse events will be monitored throughout the study and for 90 days after treatment. Dual primary end points are progression-free survival per RECIST v1.1 and overall survival. Primary end points will be assessed in the HIF arm versus control and in the CTLA arm versus control for patients with IMDC intermediate/poor status and in all patients regardless of IMDC status. Secondary end points are objective response rate, duration of response, patient-reported outcomes, and safety. The study is enrolling or planning to enroll at sites in Africa, Asia, Australia, Europe, North America, and South America:
Presented by: Brian Rini, MD, Medical Oncology Department, Vanderbilt-Ingram Cancer Center, Nashville, TN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References:


