(UroToday.com) In the on-demand poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Klara Kvorning Ternov presented an analysis of treatment-related changes in serum androgens for patients treated with abiraterone or enzalutamide in the context of a randomized controlled trial. There is a rationale for a potential difference. While enzalutamide and abiraterone acetate plus prednisone are both androgen receptor targeting treatments for metastatic castration-resistant prostate cancer (mCRPC), mechanistically they differ as abiraterone acetate inhibits the androgen production, whereas enzalutamide blocks the androgen receptor signaling.
In this investigator-initiated open-labelled randomised (1:1) phase IV trial of first-line treatment for patients with mCRPC, included patients were randomized to standard doses of enzalutamide or abiraterone acetate (EudraCT 2017-000099-27).
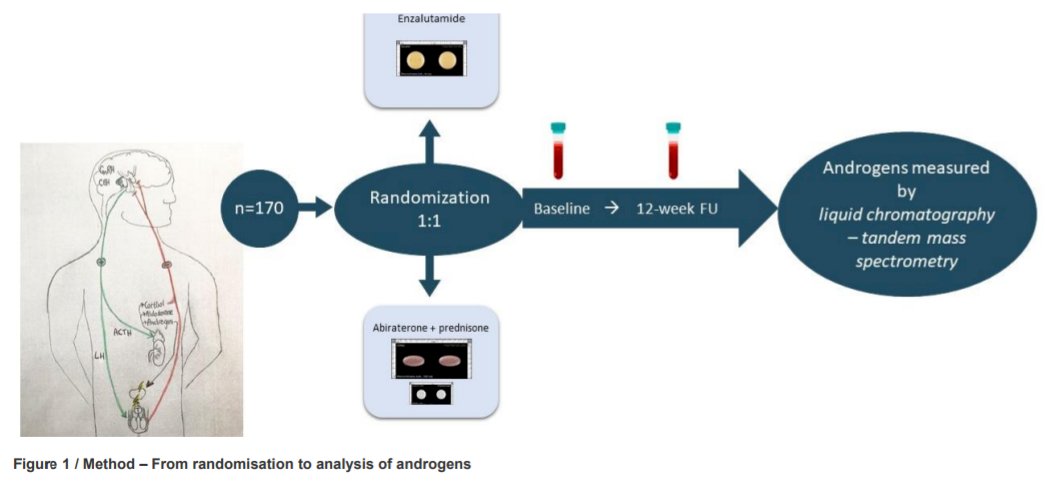
To be eligible for inclusion, patients had progressive metastatic prostate cancer on androgen deprivation therapy (testosterone <1.7nmol/L). The authors measured, as a secondary analysis, a number of laboratory parameters including fasting serum androgens, including testosterone and precursors to testosterone (androstenedione, dehydroepiandrosterone sulphate [DHEAS] and 17-Hydroxyprogesterone [17-OHP]), using the gold standard assay liquid chromatography-tandem mass spectrometry before 11 am at baseline and at 12-week post-intervention.
They used mixed models analysis to examine the treatment difference in androgens between these time periods and also considered the within-subject change for each treatment group using a paired samples t-test.
Between June 2017 to September 2019, 170 participants were randomized to receive enzalutamide (n=84 analysed) or abiraterone acetate plus prednisone (n=85 analysed). A larger decline in testosterone, androstenedione and DHEAS was found among patients who received abiraterone acetate plus prednisone than those who received enzalutamide. Among those who received enzalutamide, testosterone and DHEAS increased from baseline to week 12, whereas these decreased among those who received abiraterone acetate plus prednisone. No treatment difference was found for 17-OHP.
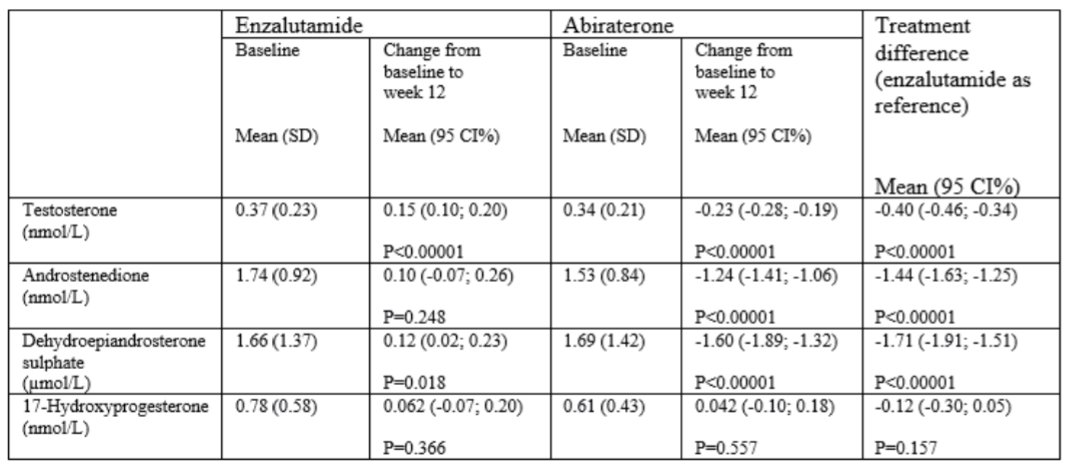
The authors conclude that these data support the different mechanism of action of these two efficacious treatment approaches in advanced prostate cancer, given different androgen profiles.
Presented by: Klara Kvorning Ternov, MD, Herlev and Gentofte University Hospital, Dept. of Urology, Herlev, Denmark


