(UroToday.com) In the on-demand poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Alicia Morgans discussed a modeling analysis of cabazitaxel compared to repeat treatment with an androgen receptor targeting agent (ARTA) among patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel and the alternative ARTA.
In the CARD trial, cabazitaxel was shown to significantly improve clinical outcomes compared to a 2nd course of treatment with ARTA in mCRPC patients who previously received docetaxel and the alternative ARTA. In particular, the authors sought to determine resource costs avoided with use of third-line cabazitaxel compared to a second ARTA.
To build their model, the authors derived model parameters from the CARD trial, medical literature, and estimates based on an assessment by 3 GU oncologists in the context of typical clinical care patterns (TCCP).
Using these model inputs, the authors created an Excel model to assess data at 6, 12, 18, and 24 months in a hypothetical patient cohort with similar characteristics to those enrolled in the CARD trial. The authors assessed the number of patients without progression and still alive, the number of hospitalization and ICU days, and costs of management of symptomatic skeletal events (SSEs), adverse events (AEs), and end-of-life care. The cost impact was modeled using costs in 2020 US$.
For each 100 patients treated, use of cabazitaxel was estimated to result in 9 more pts without progression and 17 fewer deaths, compared to use of a second course of ARTA over an 18-month period.
Additionally, use of cabazitaxel was associated with 58 fewer hospitalization days and 2 fewer ICU days and was estimated to avoid $323,095 in costs, driven by SSEs and end-of-life care. Examining specific causes of cost, the costs of SSEs, AEs, and end-of-life care were $498,909, $276,198, and $808,785, respectively, for patients modeled to receive cabazitaxel, and $627,569, $251,124, and $1,028,294, respectively, those modeled to receive a second course of ARTA.
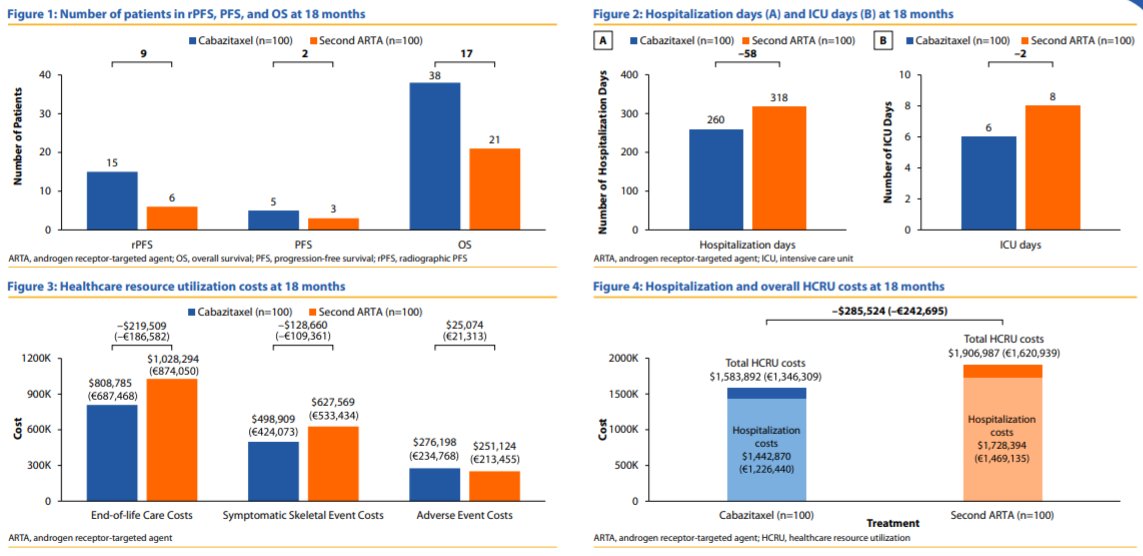
The authors therefore conclude that, based on both their analysis and the results of the CARD trial, the use of cabazitaxel as a third-line treatment after docetaxel and ARTA in mCRPC patients is predicted to result in clinical benefits (longer rPFS, OS) and fewer hospitalization and ICU days as well as a 17% decrease in overall costs, with a 21% reduction in both SSE and end-of-life care costs and a 10% increase in AE costs.
Presented by: Alicia Morgans, MD, MPH Genitourinary Medical Oncologist, Medical Director of Survivorship Program at Dana-Farber Cancer Institute, Boston, Massachusetts.


