(UroToday.com) In the on-demand poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Wassim Abida presented an analysis of the safety and efficacy of tazemetostat in combination with abiraterone and prednisone or enzalutamide in patients with metastatic castration-resistant prostate cancer (mCRPC).
Tazemetostat is an enhancer of zeste homolog (EZH2) which is approved for use in patients with epithelioid sarcoma or relapsed/refractory follicular lymphoma. However, inhibition of EZH2 has been proposed to overcome resistance to the androgen signaling inhibitors (ASIs) based on preclinical prostate cancer models, in which the combination approach led to greater tumor growth reduction than each drug alone.
The authors performed a phase Ib/II global, open-label, randomized study (NCT04179864) of tazemetostat in combination with abiraterone and prednisone or enzalutamide. The phase Ib segment enrolled adults with mCRPC following a modified 3 + 3 design. The protocol allowed prior abiraterone and prednisone, enzalutamide, first-generation anti-androgen receptor therapy, or short-course chemotherapy. Following inclusion, patients received tazemetostat escalated to 800 mg twice daily (BID) with abiraterone 1000 mg once daily (QD) + prednisone 5 mg BID or enzalutamide 160 mg QD with tazemetostat escalated to 1600 mg BID. The primary endpoints were safety, tolerability, and recommended phase II dose (RP2D) of tazemetostat for each combination. Disease control rate (DCR) was assessed at 6 months. In phase II, those treated with abiraterone + prednisone will be randomized 1:1 to tazemetostat (at RP2D) in combination with enzalutamide or to enzalutamide alone.

As of February 2021, 21 patients (of whom 7 patients received tazemetostat in combination with abiraterone and prednisone and 14 received tazemetostat in combination with enzalutamide) were enrolled. The median (range) age was 74 (53-85) years and the ECOG performance status was 0 (n=11, 52.4%) or 1 (n = 10, 47.6%).
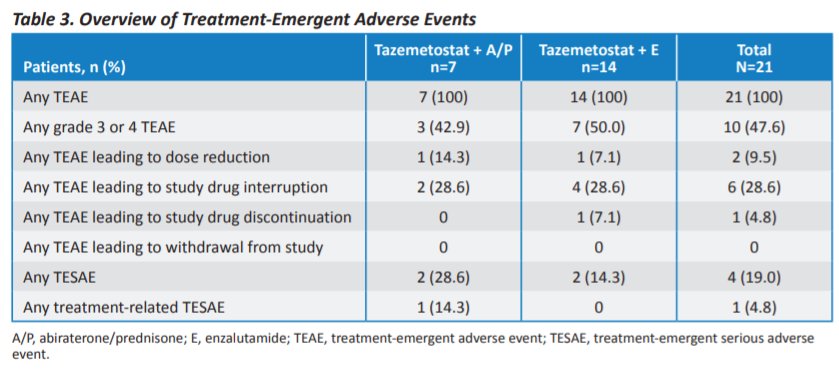
Serious treatment-emergent adverse events (TEAEs; any grade) occurred in three of 21 (14.3%) patients. Among those receiving tazemetostat in combination with abiraterone and prednisone, 1 of 7 patients (14.3%) had pneumonia and/or hyperglycemia while, among those receiving tazemetostat in combination with enzalutamide, 1/14 (7.1%) had urinary tract infection and hydronephrosis. Further, treatment-related TEAEs attributable to either study agent in each group occurred in 17 patients, of whom 7 were receiving abiraterone and prednisone and 10 were receiving enzalutamide. Further, no dose-limiting toxicities occurred in phase Ib. Thus, the RP2D for tazemetostat was 1200 mg BID in addition to standard doses of enzalutamide or abiraterone and placebo.
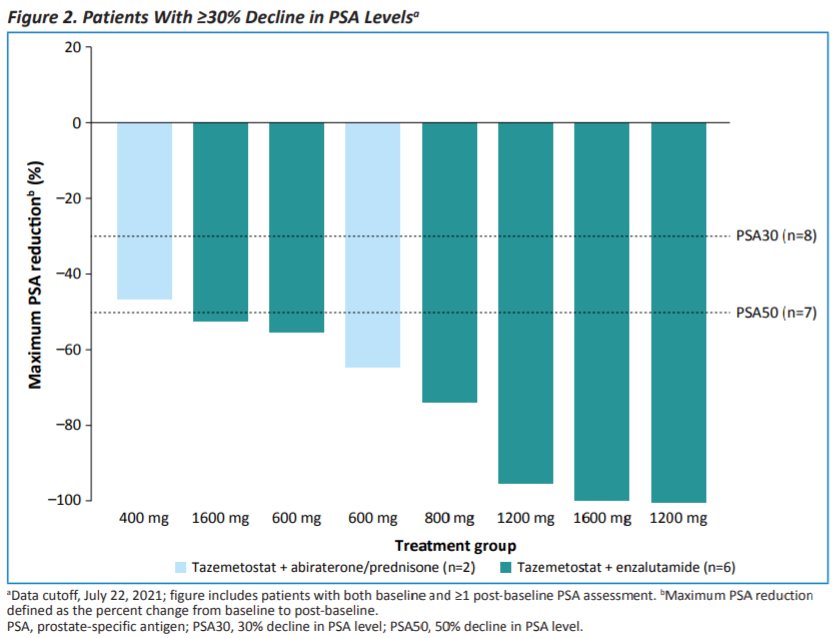
In terms of efficacy, prostate-specific antigen reduction ≥50% was observed in 7 of 20 patients, of whom 6 were treated with tazemetostat in combination with enzalutamide (of 13, 46%) and 1 was treated with tazemetostat in combination with abiraterone plus prednisone (of 7, 14%). DCR through data cutoff was 47%.
The authors, therefore, conclude that the phase Ib component of this trial demonstrates that toxicity was consistent with the known adverse events of each of these agents. Further, there is preliminary evidence of efficacy which will be further assessed in the phase II component which is actively enrolling.


