(UroToday.com) In the on-demand poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Eugene Shenderov presented results of a phase 2 trial of the anti–B7-H3 antibody, enoblituzumab, in men with localized prostate cancer. B7-H3, a member of the B7 superfamily, is highly expressed (relative to PD-L1 and PD-L2) in prostate cancer. Further, its expression is prognostic as it is associated with rapid biochemical recurrence and early metastases. Enoblituzumab is an investigational humanized Fc-optimized B7-H3–targeting antibody that induces antibody-dependent cellular cytotoxicity (ADCC).
Dr. Shenderov presented the results of a phase II single-arm neoadjuvant trial (NCT02923180) which enrolled men with operable intermediate- and high-risk localized prostate cancer (Grade Groups 3-5). These men received enoblituzumab (15 mg/kg IV weekly x 6) prior to surgery. The study sought to evaluate the safety, anti-tumor efficacy, and immunogenicity of enoblituzumab when given prior to prostatectomy.
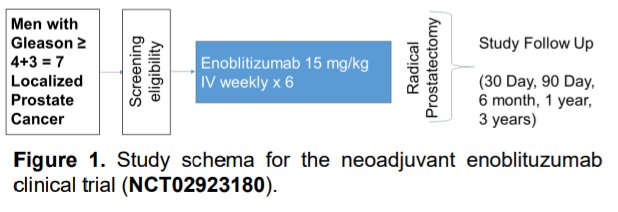
The co-primary outcomes were safety and PSA0 at 1-year post-op. Additionally, prostate glands were harvested 2 weeks after the last enoblituzumab dose and were examined for pathologic and immunologic endpoints.
The authors enrolled 32 patients. Grade 3/4 drug-related adverse events occurred in 12% of patients though none of these caused surgical delay due to AEs. A single patient developed a grade-3 infusion reaction, and one had immune myocarditis that improved with steroids.
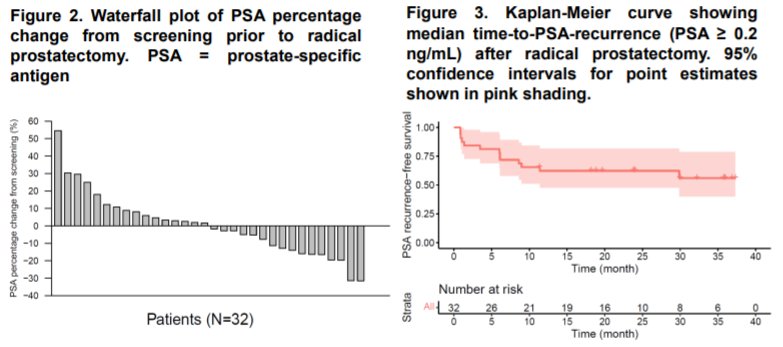
34% of patients (95% CI: 20-52%) had pre-prostatectomy PSA declines of >10%. At 1 year post-operatively, PSA0 rate was 66% (95% CI: 48-80%). The median time to PSA recurrence was not reached (95% CI: 9.4 months – NE).
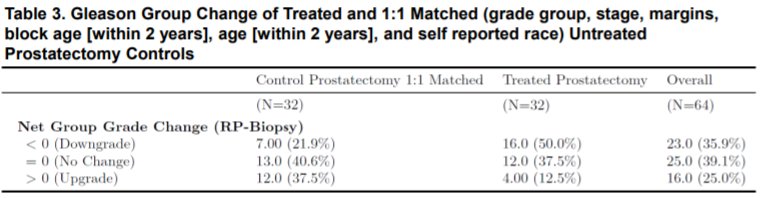
Gleason grade group upgrade, no change, and downgrade were observed in 12.5%, 37.5%, and 50% of patients respectively.
Exploratory tumor microenvironment profiling by NanoString GeoMx spatial proteomics and PanCancer IO 360 mRNA expression analysis revealed evidence of post-treatment upregulation of CD8+ T cells, PD-1/PD-L1, and immune activation (granzyme B, IFN signaling, myeloid inflammation).
Thus, the authors concluded that, in the neoadjuvant setting, inhibition of B7-H3 with enoblituzumab demonstrated favorable safety and encouraging activity in prostate cancer patients.
Presented by: Eugene Shenderov, MD, PhD, Johns Hopkins University, Baltimore, United States of America


